Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Mineralocorticoid receptor
Ligand
BDBM18161
Substrate
n/a
Meas. Tech.
ChEMBL_775983 (CHEMBL1912981)
IC50
2100±n/a nM
Citation
 Nagata, N; Miyakawa, M; Amano, S; Furuya, K; Yamamoto, N; Nejishima, H; Inoguchi, K Tetrahydroquinolines as a novel series of nonsteroidal selective androgen receptor modulators: structural requirements for better physicochemical and biological properties. Bioorg Med Chem Lett 21:6310-3 (2011) [PubMed] Article
Nagata, N; Miyakawa, M; Amano, S; Furuya, K; Yamamoto, N; Nejishima, H; Inoguchi, K Tetrahydroquinolines as a novel series of nonsteroidal selective androgen receptor modulators: structural requirements for better physicochemical and biological properties. Bioorg Med Chem Lett 21:6310-3 (2011) [PubMed] Article More Info.:
Target
Name:
Mineralocorticoid receptor
Synonyms:
MCR_RAT | MR | Mineralocorticoid Receptor (MR) | Mineralocorticoid receptor | Mlr | Nr3c2 | Nuclear receptor subfamily 3 group C member 2 | mineralocorticoid
Type:
Enzyme Catalytic Domain
Mol. Mass.:
106748.15
Organism:
RAT
Description:
mineralocorticoid 0 RAT::P22199
Residue:
981
Sequence:
METKGYHSLPEGLDMERRWSQVSQTLERSSLGPAERTTENNYMEIVNVSCVSGAIPNNSTQGSSKEKHELLPYIQQDNSRSGILPSDIKTELESKELSATVAESMGLYMDSVRDAEYTYDQQNQQGSLSPTKIYQNMEQLVKFYKENGHRSSTLSAMSRPLRSFMPDSAASMNGGALRAIVKSPIICHEKSSSVSSPLNMASSVCSPVGINSMSSSTTSFGSFPVHSPITQGTSLTCSPSVENRGSRSHSPTHASNVGSPLSSPLSSMKSPISSPPSHCSVKSPVSSPNNVPLRSSVSSPANLNNSRCSVSSPSNNTNNRSTLSSPTASTVGSIGSPISNAFSYATSGASAGAGAIQDVVPSPDTHEKGAHDVPFPKTEEVEKAISNGVTGPLNIVQYIKSEPDGAFSSSCLGGNSKISPSSPFSVPIKQESSKHSCSGASFKGNPTVNPFPFMDGSYFSFMDDKDYYSLSGILGPPVPGFDGSCEDSAFPVGIKQEPDDGSYYPEASIPSSAIVGVNSGGQSFHYRIGAQGTISLSRSPRDQSFQHLSSFPPVNTLVESWKPHGDLSSRRSDGYPVLEYIPENVSSSTLRSVSTGSSRPSKICLVCGDEASGCHYGVVTCGSCKVFFKRAVEGQHNYLCAGRNDCIIDKIRRKNCPACRLQKCLQAGMNLGARKSKKLGKLKGLHEEQPQQPPPPPPQSPEEGTTYIAPTKEPSVNSALVPQLTSITHALTPSPAMILENIEPETVYAGYDNSKPDTAESLLSTLNRLAAKQMIQVVKWAKVLPGFKNLPLEDQITLIQYSWMCLSSFALSWRSYKHTNSQLLYFAPDLVFNEEKMHQSAMYELCQGMRQISLQFVRLQLTFEEYSIMKVLLLLSTVPKDGLKSQAAFEEMRTNYIKELRKMVTKCPNSSGQSWQRFYQLTKLLDSMHDLVSDLLEFCFYTFRESQALKVEFPAMLVEIITDQLPKVESGNAKPLYFHRK
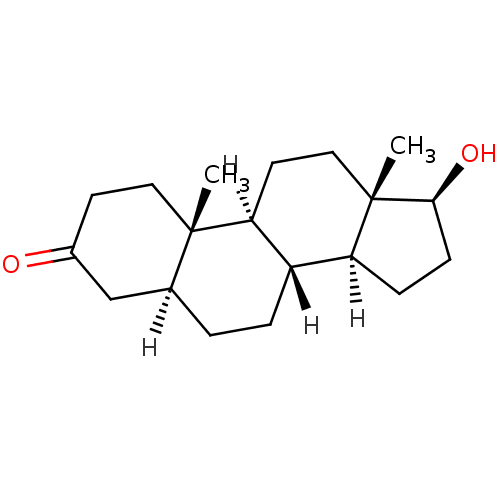
Inhibitor
Name:
BDBM18161
Synonyms:
(1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-5-one | (5alpha,17beta)-17-hydroxyandrostan-3-one | CHEMBL27769 | DHT | Dihydrotestosterone | [3H]DHT
Type:
Steroid
Emp. Form.:
C19H30O2
Mol. Mass.:
290.4403
SMILES:
[H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r|