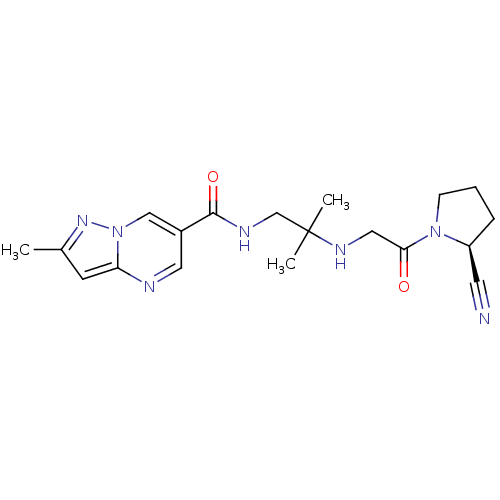
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Dipeptidyl peptidase 8
Ligand
BDBM50359366
Substrate
n/a
Meas. Tech.
ChEMBL_793025 (CHEMBL1932591)
IC50
68±n/a nM
Citation
 Kato, N; Oka, M; Murase, T; Yoshida, M; Sakairi, M; Yamashita, S; Yasuda, Y; Yoshikawa, A; Hayashi, Y; Makino, M; Takeda, M; Mirensha, Y; Kakigami, T Discovery and pharmacological characterization of N-[2-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl}amino)-2-methylpropyl]-2-methylpyrazolo[1,5-a]pyrimidine-6-carboxamide hydrochloride (anagliptin hydrochloride salt) as a potent and selective DPP-IV inhibitor. Bioorg Med Chem 19:7221-7 (2011) [PubMed] Article
Kato, N; Oka, M; Murase, T; Yoshida, M; Sakairi, M; Yamashita, S; Yasuda, Y; Yoshikawa, A; Hayashi, Y; Makino, M; Takeda, M; Mirensha, Y; Kakigami, T Discovery and pharmacological characterization of N-[2-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl}amino)-2-methylpropyl]-2-methylpyrazolo[1,5-a]pyrimidine-6-carboxamide hydrochloride (anagliptin hydrochloride salt) as a potent and selective DPP-IV inhibitor. Bioorg Med Chem 19:7221-7 (2011) [PubMed] Article More Info.:
Target
Name:
Dipeptidyl peptidase 8
Synonyms:
DPP8 | DPP8_HUMAN | DPRP-1 | DPRP1 | Dipeptidyl peptidase 8 (DPP-8) | Dipeptidyl peptidase 8 (DPP8) | Dipeptidyl peptidase 8/9 | Dipeptidyl peptidase IV-related protein 1 | Dipeptidyl peptidase VIII | Dipeptidyl peptidase VIII (DDP-VIII) | Prolyl dipeptidase DPP8
Type:
Enzyme
Mol. Mass.:
103342.62
Organism:
Homo sapiens (Human)
Description:
Q6V1X1
Residue:
898
Sequence:
MWKRSEQMKIKSGKCNMAAAMETEQLGVEIFETADCEENIESQDRPKLEPFYVERYSWSQLKKLLADTRKYHGYMMAKAPHDFMFVKRNDPDGPHSDRIYYLAMSGENRENTLFYSEIPKTINRAAVLMLSWKPLLDLFQATLDYGMYSREEELLRERKRIGTVGIASYDYHQGSGTFLFQAGSGIYHVKDGGPQGFTQQPLRPNLVETSCPNIRMDPKLCPADPDWIAFIHSNDIWISNIVTREERRLTYVHNELANMEEDARSAGVATFVLQEEFDRYSGYWWCPKAETTPSGGKILRILYEENDESEVEIIHVTSPMLETRRADSFRYPKTGTANPKVTFKMSEIMIDAEGRIIDVIDKELIQPFEILFEGVEYIARAGWTPEGKYAWSILLDRSQTRLQIVLISPELFIPVEDDVMERQRLIESVPDSVTPLIIYEETTDIWINIHDIFHVFPQSHEEEIEFIFASECKTGFRHLYKITSILKESKYKRSSGGLPAPSDFKCPIKEEIAITSGEWEVLGRHGSNIQVDEVRRLVYFEGTKDSPLEHHLYVVSYVNPGEVTRLTDRGYSHSCCISQHCDFFISKYSNQKNPHCVSLYKLSSPEDDPTCKTKEFWATILDSAGPLPDYTPPEIFSFESTTGFTLYGMLYKPHDLQPGKKYPTVLFIYGGPQVQLVNNRFKGVKYFRLNTLASLGYVVVVIDNRGSCHRGLKFEGAFKYKMGQIEIDDQVEGLQYLASRYDFIDLDRVGIHGWSYGGYLSLMALMQRSDIFRVAIAGAPVTLWIFYDTGYTERYMGHPDQNEQGYYLGSVAMQAEKFPSEPNRLLLLHGFLDENVHFAHTSILLSFLVRAGKPYDLQIYPQERHSIRVPESGEHYELHLLHYLQENLGSRIAALKVI