Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Neprilysin
Ligand
BDBM50251742
Substrate
n/a
Meas. Tech.
ChEBML_64519
Ki
4±n/a nM
Citation
 Elliott, RL; Marks, N; Berg, MJ; Portoghese, PS Synthesis and biological evaluation of phosphonamidate peptide inhibitors of enkephalinase and angiotensin-converting enzyme. J Med Chem 28:1208-16 (1985) [PubMed] Article
Elliott, RL; Marks, N; Berg, MJ; Portoghese, PS Synthesis and biological evaluation of phosphonamidate peptide inhibitors of enkephalinase and angiotensin-converting enzyme. J Med Chem 28:1208-16 (1985) [PubMed] Article More Info.:
Target
Name:
Neprilysin
Synonyms:
Mme | NEP_RAT | Neprilysin | Neutral Endopeptidase (NEP) | Neutral endopeptidase 24.11
Type:
Protein
Mol. Mass.:
85789.59
Organism:
Rattus norvegicus (Rat)
Description:
P07861
Residue:
750
Sequence:
MGRSESQMDITDINAPKPKKKQRWTPLEISLSVLVLLLTIIAVTMIALYATYDDGICKSSDCIKSAARLIQNMDASAEPCTDFFKYACGGWLKRNVIPETSSRYSNFDILRDELEVILKDVLQEPKTEDIVAVQKAKTLYRSCINESAIDSRGGQPLLTLLPDIYGWPVASQNWEQTYGTSWTAEKSIAQLNSKYGKKVLINFFVGTDDKNSTQHIIHFDQPRLGLPSRDYYECTGIYKEACTAYVDFMISVARLIRQEQRLPIDENQLSLEMNKVMELEKEIANATTKPEDRNDPMLLYNKMTLAKLQNNFSLEINGKPFSWSNFTNEIMSTVNINIQNEEEVVVYAPEYLTKLKPILTKYSPRDLQNLMSWRFIMDLVSSLSRNYKESRNAFRKALYGTTSETATWRRCANYVNGNMENAVGRLYVEAAFAGESKHVVEDLIAQIREVFIQTLDDLTWMDAETKKKAEEKALAIKERIGYPDDIISNENKLNNEYLELNYKEEEYFENIIQNLKFSQSKQLKKLREKVDKDEWISGAAVVNAFYSSGRNQIVFPAGILQPPFFSARQSNSLNYGGIGMVIGHEITHGFDDNGRNFNKDGDLVDWWTQQSANNFKDQSQCMVYQYGNFTWDLAGGQHLNGINTLGENIADNGGIGQAYRAYQNYVKKNGEEKLLPGLDLNHKQLFFLNFAQVWCGTYRPEYAVNSIKTDVHSPGNFRIIGTLQNSAEFADAFHCRKNSYMNPERKCRVW
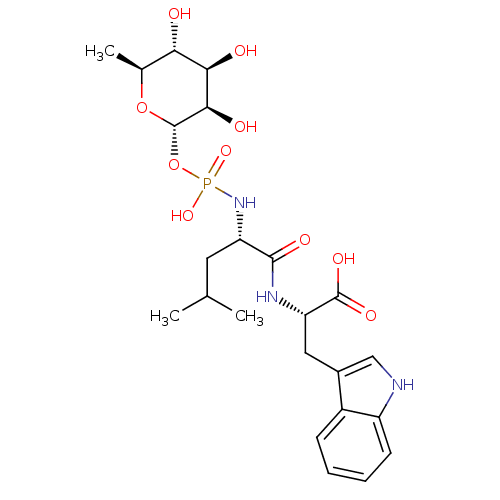
Inhibitor
Name:
BDBM50251742
Synonyms:
(3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-2H-pyran-2-yl hydrogen (S)-1-((S)-1-(1H-indol-3-yl)-3-oxobutan-2-ylamino)-4-methyl-1-oxopentan-2-ylphosphoramidate | (S)-2-{(R)-2-[Hydroxy-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-3-(1H-indol-3-yl)-propionic acid | (S)-2-{(S)-2-[Hydroxy-((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-3-(1H-indol-3-yl)-propionic acid | (S)-2-{(S)-2-[Hydroxy-((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-3-(1H-indol-3-yl)-propionic acid | (S)-2-{(S)-2-[Hydroxy-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-1-oxo-pentylamino}-3-(1H-indol-3-yl)-propionic acid | (S)-2-{(S)-2-[Hydroxy-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-3-(1H-indol-3-yl)-propionic acid | (S)-3-(1-Hydroxy-1H-indol-3-yl)-2-{(S)-2-[hydroxy-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-propionic acid | (phosphoramidon) 2-{2-[Hydroxy-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-3-(1H-indol-3-yl)-propionic acid | 2-{2-[Hydroxy-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-3-(1H-indol-3-yl)-propionic acid | 2-{2-[Hydroxy-(3,4,6-trihydroxy-5-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-3-(1H-indol-3-yl)-propionic acid( Phosphoramidon) | CHEMBL479579 | N-alpha-L-rhamnopyranosyloxy(hydroxyphosphinyl)-L-Leucyl-L-Tryptophan | Phosphoramidon | Phosporamidon | phosphramidon
Type:
Small organic molecule
Emp. Form.:
C23H34N3O10P
Mol. Mass.:
543.5039
SMILES:
CC(C)C[C@H](NP(O)(=O)O[C@@H]1O[C@@H](C)[C@H](O)[C@@H](O)[C@H]1O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r|