Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
3-hydroxy-3-methylglutaryl-coenzyme A reductase
Ligand
BDBM50007268
Substrate
n/a
Meas. Tech.
ChEBML_78556
IC50
>1000±n/a nM
Citation
 Jendralla, H; Granzer, E; von Kerekjarto, B; Krause, R; Schacht, U; Baader, E; Bartmann, W; Beck, G; Bergmann, A; Kesseler, K Synthesis and biological activity of new HMG-CoA reductase inhibitors. 3. Lactones of 6-phenoxy-3,5-dihydroxyhexanoic acids. J Med Chem 34:2962-83 (1991) [PubMed] Article
Jendralla, H; Granzer, E; von Kerekjarto, B; Krause, R; Schacht, U; Baader, E; Bartmann, W; Beck, G; Bergmann, A; Kesseler, K Synthesis and biological activity of new HMG-CoA reductase inhibitors. 3. Lactones of 6-phenoxy-3,5-dihydroxyhexanoic acids. J Med Chem 34:2962-83 (1991) [PubMed] Article More Info.:
Target
Name:
3-hydroxy-3-methylglutaryl-coenzyme A reductase
Synonyms:
3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase | 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA) | HMDH_HUMAN | HMG-CoA Reductase | HMG-CoA reductase (HMGR) | HMGCR
Type:
Enzyme
Mol. Mass.:
97477.10
Organism:
Homo sapiens (Human)
Description:
P04035
Residue:
888
Sequence:
MLSRLFRMHGLFVASHPWEVIVGTVTLTICMMSMNMFTGNNKICGWNYECPKFEEDVLSSDIIILTITRCIAILYIYFQFQNLRQLGSKYILGIAGLFTIFSSFVFSTVVIHFLDKELTGLNEALPFFLLLIDLSRASTLAKFALSSNSQDEVRENIARGMAILGPTFTLDALVECLVIGVGTMSGVRQLEIMCCFGCMSVLANYFVFMTFFPACVSLVLELSRESREGRPIWQLSHFARVLEEEENKPNPVTQRVKMIMSLGLVLVHAHSRWIADPSPQNSTADTSKVSLGLDENVSKRIEPSVSLWQFYLSKMISMDIEQVITLSLALLLAVKYIFFEQTETESTLSLKNPITSPVVTQKKVPDNCCRREPMLVRNNQKCDSVEEETGINRERKVEVIKPLVAETDTPNRATFVVGNSSLLDTSSVLVTQEPEIELPREPRPNEECLQILGNAEKGAKFLSDAEIIQLVNAKHIPAYKLETLMETHERGVSIRRQLLSKKLSEPSSLQYLPYRDYNYSLVMGACCENVIGYMPIPVGVAGPLCLDEKEFQVPMATTEGCLVASTNRGCRAIGLGGGASSRVLADGMTRGPVVRLPRACDSAEVKAWLETSEGFAVIKEAFDSTSRFARLQKLHTSIAGRNLYIRFQSRSGDAMGMNMISKGTEKALSKLHEYFPEMQILAVSGNYCTDKKPAAINWIEGRGKSVVCEAVIPAKVVREVLKTTTEAMIEVNINKNLVGSAMAGSIGGYNAHAANIVTAIYIACGQDAAQNVGSSNCITLMEASGPTNEDLYISCTMPSIEIGTVGGGTNLLPQQACLQMLGVQGACKDNPGENARQLARIVCGTVMAGELSLMAALAAGHLVKSHMIHNRSKINLQDLQGACTKKTA
Inhibitor
Name:
BDBM50007268
Synonyms:
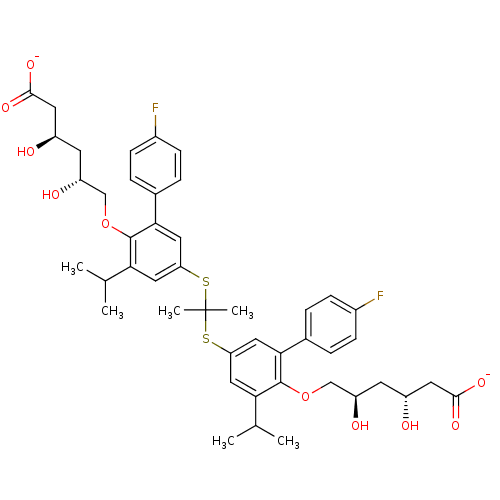
6-[4-{1-[4-[5-carboxylato-2,4-dihydroxy-(2R,4R)-pentyloxy]-3-(4-fluorophenyl)-5-isopropylphenylsulfanyl]-1-methylethylsulfanyl}-2-(4-fluorophenyl)-6-isopropylphenoxy]-3,5-dihydroxy-(3R,5R)-hexanoate;2Sodium ion | CHEMBL321076
Type:
Small organic molecule
Emp. Form.:
C45H52F2O10S2
Mol. Mass.:
855.016
SMILES:
CC(C)c1cc(SC(C)(C)Sc2cc(C(C)C)c(OC[C@H](O)C[C@@H](O)CC([O-])=O)c(c2)-c2ccc(F)cc2)cc(-c2ccc(F)cc2)c1OC[C@H](O)C[C@@H](O)CC([O-])=O