Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Voltage-dependent L-type calcium channel subunit alpha-1D
Ligand
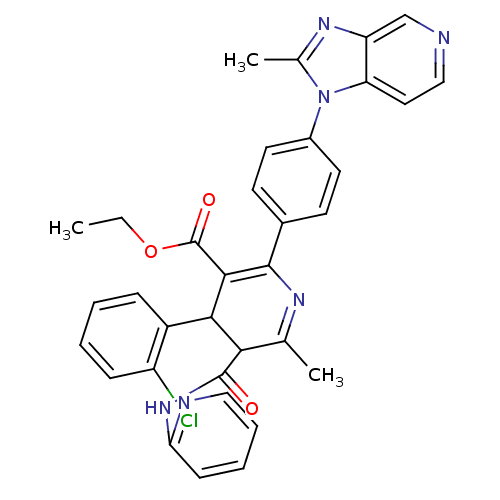
BDBM50004633
Substrate
n/a
Meas. Tech.
ChEMBL_154991 (CHEMBL765343)
IC50
6600±n/a nM
Citation
More Info.:
Target
Name:
Voltage-dependent L-type calcium channel subunit alpha-1D
Synonyms:
CAC1D_HUMAN | CACH3 | CACN4 | CACNA1D | CACNL1A2 | CCHL1A2 | Calcium channel, L type, alpha-1 polypeptide, isoform 2 | Voltage-dependent L-type calcium channel subunit alpha-1D | Voltage-gated L-type calcium channel | Voltage-gated L-type calcium channel alpha-1D subunit | Voltage-gated calcium channel | Voltage-gated calcium channel subunit alpha Cav1.3
Type:
PROTEIN
Mol. Mass.:
245144.84
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1350501
Residue:
2161
Sequence:
MMMMMMMKKMQHQRQQQADHANEANYARGTRLPLSGEGPTSQPNSSKQTVLSWQAAIDAARQAKAAQTMSTSAPPPVGSLSQRKRQQYAKSKKQGNSSNSRPARALFCLSLNNPIRRACISIVEWKPFDIFILLAIFANCVALAIYIPFPEDDSNSTNHNLEKVEYAFLIIFTVETFLKIIAYGLLLHPNAYVRNGWNLLDFVIVIVGLFSVILEQLTKETEGGNHSSGKSGGFDVKALRAFRVLRPLRLVSGVPSLQVVLNSIIKAMVPLLHIALLVLFVIIIYAIIGLELFIGKMHKTCFFADSDIVAEEDPAPCAFSGNGRQCTANGTECRSGWVGPNGGITNFDNFAFAMLTVFQCITMEGWTDVLYWMNDAMGFELPWVYFVSLVIFGSFFVLNLVLGVLSGEFSKEREKAKARGDFQKLREKQQLEEDLKGYLDWITQAEDIDPENEEEGGEEGKRNTSMPTSETESVNTENVSGEGENRGCCGSLCQAISKSKLSRRWRRWNRFNRRRCRAAVKSVTFYWLVIVLVFLNTLTISSEHYNQPDWLTQIQDIANKVLLALFTCEMLVKMYSLGLQAYFVSLFNRFDCFVVCGGITETILVELEIMSPLGISVFRCVRLLRIFKVTRHWTSLSNLVASLLNSMKSIASLLLLLFLFIIIFSLLGMQLFGGKFNFDETQTKRSTFDNFPQALLTVFQILTGEDWNAVMYDGIMAYGGPSSSGMIVCIYFIILFICGNYILLNVFLAIAVDNLADAESLNTAQKEEAEEKERKKIARKESLENKKNNKPEVNQIANSDNKVTIDDYREEDEDKDPYPPCDVPVGEEEEEEEEDEPEVPAGPRPRRISELNMKEKIAPIPEGSAFFILSKTNPIRVGCHKLINHHIFTNLILVFIMLSSAALAAEDPIRSHSFRNTILGYFDYAFTAIFTVEILLKMTTFGAFLHKGAFCRNYFNLLDMLVVGVSLVSFGIQSSAISVVKILRVLRVLRPLRAINRAKGLKHVVQCVFVAIRTIGNIMIVTTLLQFMFACIGVQLFKGKFYRCTDEAKSNPEECRGLFILYKDGDVDSPVVRERIWQNSDFNFDNVLSAMMALFTVSTFEGWPALLYKAIDSNGENIGPIYNHRVEISIFFIIYIIIVAFFMMNIFVGFVIVTFQEQGEKEYKNCELDKNQRQCVEYALKARPLRRYIPKNPYQYKFWYVVNSSPFEYMMFVLIMLNTLCLAMQHYEQSKMFNDAMDILNMVFTGVFTVEMVLKVIAFKPKGYFSDAWNTFDSLIVIGSIIDVALSEADPTESENVPVPTATPGNSEESNRISITFFRLFRVMRLVKLLSRGEGIRTLLWTFIKSFQALPYVALLIAMLFFIYAVIGMQMFGKVAMRDNNQINRNNNFQTFPQAVLLLFRCATGEAWQEIMLACLPGKLCDPESDYNPGEEYTCGSNFAIVYFISFYMLCAFLIINLFVAVIMDNFDYLTRDWSILGPHHLDEFKRIWSEYDPEAKGRIKHLDVVTLLRRIQPPLGFGKLCPHRVACKRLVAMNMPLNSDGTVMFNATLFALVRTALKIKTEGNLEQANEELRAVIKKIWKKTSMKLLDQVVPPAGDDEVTVGKFYATFLIQDYFRKFKKRKEQGLVGKYPAKNTTIALQAGLRTLHDIGPEIRRAISCDLQDDEPEETKREEEDDVFKRNGALLGNHVNHVNSDRRDSLQQTNTTHRPLHVQRPSIPPASDTEKPLFPPAGNSVCHNHHNHNSIGKQVPTSTNANLNNANMSKAAHGKRPSIGNLEHVSENGHHSSHKHDREPQRRSSVKRTRYYETYIRSDSGDEQLPTICREDPEIHGYFRDPHCLGEQEYFSSEECYEDDSSPTWSRQNYGYYSRYPGRNIDSERPRGYHHPQGFLEDDDSPVCYDSRRSPRRRLLPPTPASHRRSSFNFECLRRQSSQEEVPSSPIFPHRTALPLHLMQQQIMAVAGLDSSKAQKYSPSHSTRSWATPPATPPYRDWTPCYTPLIQVEQSEALDQVNGSLPSLHRSSWYTDEPDISYRTFTPASLTVPSSFRNKNSDKQRSADSLVEAVLISEGLGRYARDPKFVSATKHEIADACDLTIDEMESAASTLLNGNVRPRANGDVGPLSHRQDYELQDFGPGYSDEEPDPGRDEEDLADEMICITTL
Inhibitor
Name:
BDBM50004633
Synonyms:
(S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-imidazo[4,5-c]pyridin-1-yl)-phenyl]-5-(pyridin-2-ylcarbamoyl)-1,4-dihydro-pyridine-3-carboxylic acid ethyl ester | 4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-imidazo[4,5-c]pyridin-1-yl)-phenyl]-5-(pyridin-2-ylcarbamoyl)-1,4-dihydro-pyridine-3-carboxylic acid ethyl ester | 4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-imidazo[4,5-c]pyridin-1-yl)-phenyl]-5-(pyridin-2-ylcarbamoyl)-nicotinic acid ethyl ester | CHEMBL29067 | UK-74505
Type:
Small organic molecule
Emp. Form.:
C34H29ClN6O3
Mol. Mass.:
605.085
SMILES:
CCOC(=O)C1=C(N=C(C)C(C1c1ccccc1Cl)C(=O)Nc1ccccn1)c1ccc(cc1)-n1c(C)nc2cnccc12 |t:5,7|