Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Polyunsaturated fatty acid 5-lipoxygenase
Ligand
BDBM50000540
Substrate
n/a
Meas. Tech.
ChEBML_98492
EC50
6800±n/a nM
Citation
 Greco, MN; Hageman, WE; Powell, ET; Tighe, JJ; Persico, FJ Benzothiazole hydroxy ureas as inhibitors of 5-lipoxygenase: use of the hydroxyurea moiety as a replacement for hydroxamic acid. J Med Chem 35:3180-3 (1992) [PubMed] Article
Greco, MN; Hageman, WE; Powell, ET; Tighe, JJ; Persico, FJ Benzothiazole hydroxy ureas as inhibitors of 5-lipoxygenase: use of the hydroxyurea moiety as a replacement for hydroxamic acid. J Med Chem 35:3180-3 (1992) [PubMed] Article More Info.:
Target
Name:
Polyunsaturated fatty acid 5-lipoxygenase
Synonyms:
5-LO | 5-Lipo-oxygenase (5-LOX) | 5-Lipoxygenase (5-LO) | 5-Lipoxygenase (LOX) | 5-Lipoygenase | 5-lipoxygenase/FLAP | ALOX5 | Arachidonate 5-lipoxygenase | LOG5 | LOX5_HUMAN
Type:
Enzyme
Mol. Mass.:
77972.74
Organism:
Homo sapiens (Human)
Description:
Recombinant protein was purified from E. coli lysate. After ammonium sulfate precipitation and subsequent steps, the supernatant (S100) was used for 5-LO activity assay.
Residue:
674
Sequence:
MPSYTVTVATGSQWFAGTDDYIYLSLVGSAGCSEKHLLDKPFYNDFERGAVDSYDVTVDEELGEIQLVRIEKRKYWLNDDWYLKYITLKTPHGDYIEFPCYRWITGDVEVVLRDGRAKLARDDQIHILKQHRRKELETRQKQYRWMEWNPGFPLSIDAKCHKDLPRDIQFDSEKGVDFVLNYSKAMENLFINRFMHMFQSSWNDFADFEKIFVKISNTISERVMNHWQEDLMFGYQFLNGCNPVLIRRCTELPEKLPVTTEMVECSLERQLSLEQEVQQGNIFIVDFELLDGIDANKTDPCTLQFLAAPICLLYKNLANKIVPIAIQLNQIPGDENPIFLPSDAKYDWLLAKIWVRSSDFHVHQTITHLLRTHLVSEVFGIAMYRQLPAVHPIFKLLVAHVRFTIAINTKAREQLICECGLFDKANATGGGGHVQMVQRAMKDLTYASLCFPEAIKARGMESKEDIPYYFYRDDGLLVWEAIRTFTAEVVDIYYEGDQVVEEDPELQDFVNDVYVYGMRGRKSSGFPKSVKSREQLSEYLTVVIFTASAQHAAVNFGQYDWCSWIPNAPPTMRAPPPTAKGVVTIEQIVDTLPDRGRSCWHLGAVWALSQFQENELFLGMYPEEHFIEKPVKEAMARFRKNLEAIVSVIAERNKKKQLPYYYLSPDRIPNSVAI
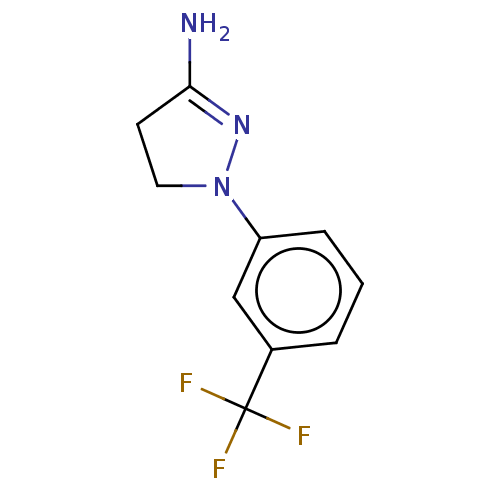
Inhibitor
Name:
BDBM50000540
Synonyms:
1-(3-Trifluoromethyl-phenyl)-4,5-dihydro-1H-pyrazol-3-ylamine | 1-(3-Trifluoromethyl-phenyl)-4,5-dihydro-1H-pyrazol-3-ylamine (BW-755C) | 1-(3-Trifluoromethyl-phenyl)-4,5-dihydro-1H-pyrazol-3-ylamine(BW 755 C) | 1-(3-Trifluoromethyl-phenyl)-4,5-dihydro-1H-pyrazol-3-ylamine(BW 755C) | 1-(3-trifluoromethylphenyl)-4,5-dihydro-1H-3-pyrazolamine | BW-755C | CHEMBL274642
Type:
Small organic molecule
Emp. Form.:
C10H10F3N3
Mol. Mass.:
229.2017
SMILES:
NC1=NN(CC1)c1cccc(c1)C(F)(F)F |t:1|