Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Gastrin/cholecystokinin type B receptor
Ligand
BDBM50005821
Substrate
n/a
Meas. Tech.
ChEBML_48256
IC50
19500±n/a nM
Citation
 Fincham, CI; Higginbottom, M; Hill, DR; Horwell, DC; O'Toole, JC; Ratcliffe, GS; Rees, DC; Roberts, E Amide bond replacements incorporated into CCK-B selective"dipeptoids". J Med Chem 35:1472-84 (1992) [PubMed] Article
Fincham, CI; Higginbottom, M; Hill, DR; Horwell, DC; O'Toole, JC; Ratcliffe, GS; Rees, DC; Roberts, E Amide bond replacements incorporated into CCK-B selective"dipeptoids". J Med Chem 35:1472-84 (1992) [PubMed] Article More Info.:
Target
Name:
Gastrin/cholecystokinin type B receptor
Synonyms:
Cckbr | Cholecystokinin A | Cholecystokinin receptor | GASR_MOUSE | Gastrin/cholecystokinin type B receptor
Type:
Enzyme Catalytic Domain
Mol. Mass.:
49196.59
Organism:
MOUSE
Description:
Cholecystokinin A CCKBR MOUSE::P56481
Residue:
453
Sequence:
MDLLKLNRSLQGPGPGSGSSLCRPGVSLLNSSSAGNLSCETPRIRGTGTRELELTIRITLYAVIFLMSVGGNVLIIVVLGLSRRLRTVTNAFLLSLAVSDLLLAVACMPFTLLPNLMGTFIFGTVICKAVSYLMGVSVSVSTLNLAAIALERYSAICRPLQARVWQTRSHAARVILATWLLSGLLMVPYPVYTVVQPVGPRILQCMHLWPSERVQQMWSVLLLILLFFIPGVVMAVAYGLISRELYLGLRFDGDNDSETQSRVRNQGGLPGGAAAPGPVHQNGGCRHVTSLTGEDSDGCYVQLPRSRLEMTTLTTPTTGPGPGPRPNQAKLLAKKRVVRMLLVIVLLFFVCWLPVYSANTWRAFDGPGARRALAGAPISFIHLLSYTSACANPLVYCFMHRRFRQACLDTCARCCPRPPRARPRPLPDEDPPTPSIASLSRLSYTTISTLGPG
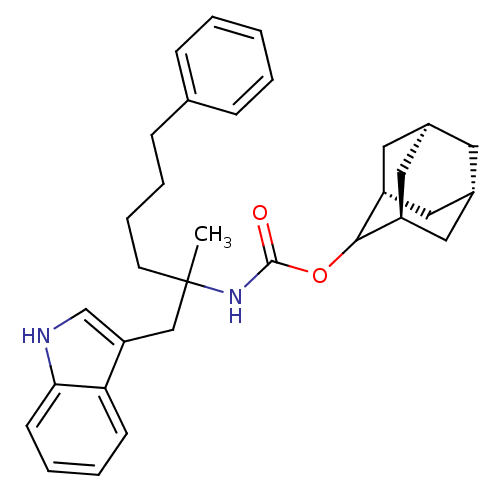
Inhibitor
Name:
BDBM50005821
Synonyms:
CHEMBL286093 | [1-(1H-Indol-3-ylmethyl)-1-methyl-5-phenyl-pentyl]-carbamic acid adamantan-2-yl ester;0.25H2O
Type:
Small organic molecule
Emp. Form.:
C32H40N2O2
Mol. Mass.:
484.6722
SMILES:
CC(CCCCc1ccccc1)(Cc1c[nH]c2ccccc12)NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2 |wU:31.39,wD:29.31,33.35,27.40,TLB:25:26:32:29.34.30,THB:28:27:32:29.34.30,28:29:32:26.27.35,30:31:26:29.34.28,(13.87,-8.6,;13.1,-7.26,;14.63,-7.15,;15.49,-8.41,;17.01,-8.32,;17.88,-9.59,;19.26,-8.86,;20.54,-9.7,;21.92,-8.99,;21.98,-7.45,;20.67,-6.62,;19.31,-7.35,;11.85,-6.36,;12.01,-4.82,;10.85,-3.82,;11.48,-2.41,;13,-2.54,;14.15,-1.52,;15.6,-1.97,;15.94,-3.5,;14.8,-4.53,;13.34,-4.05,;12.33,-8.6,;10.79,-8.58,;10.04,-7.23,;10.01,-9.89,;8.47,-9.86,;8.47,-11.39,;7.44,-12.65,;6.04,-12.09,;4.53,-12.51,;5.74,-11.24,;5.72,-9.76,;7.07,-9.28,;6.03,-10.5,;7.06,-11.72,)|