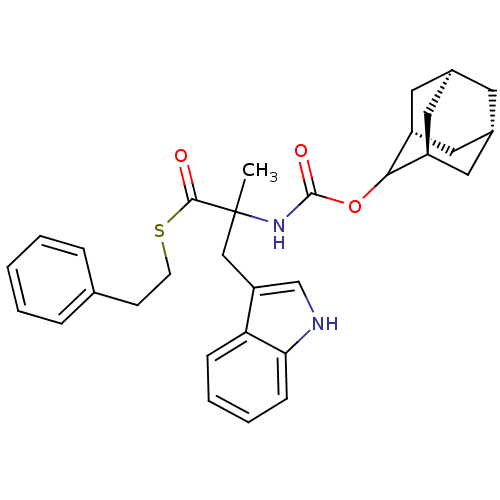
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Gastrin/cholecystokinin type B receptor
Ligand
BDBM50005828
Substrate
n/a
Meas. Tech.
ChEBML_48256
IC50
1190±n/a nM
Citation
 Fincham, CI; Higginbottom, M; Hill, DR; Horwell, DC; O'Toole, JC; Ratcliffe, GS; Rees, DC; Roberts, E Amide bond replacements incorporated into CCK-B selective"dipeptoids". J Med Chem 35:1472-84 (1992) [PubMed] Article
Fincham, CI; Higginbottom, M; Hill, DR; Horwell, DC; O'Toole, JC; Ratcliffe, GS; Rees, DC; Roberts, E Amide bond replacements incorporated into CCK-B selective"dipeptoids". J Med Chem 35:1472-84 (1992) [PubMed] Article More Info.:
Target
Name:
Gastrin/cholecystokinin type B receptor
Synonyms:
Cckbr | Cholecystokinin A | Cholecystokinin receptor | GASR_MOUSE | Gastrin/cholecystokinin type B receptor
Type:
Enzyme Catalytic Domain
Mol. Mass.:
49196.59
Organism:
MOUSE
Description:
Cholecystokinin A CCKBR MOUSE::P56481
Residue:
453
Sequence:
MDLLKLNRSLQGPGPGSGSSLCRPGVSLLNSSSAGNLSCETPRIRGTGTRELELTIRITLYAVIFLMSVGGNVLIIVVLGLSRRLRTVTNAFLLSLAVSDLLLAVACMPFTLLPNLMGTFIFGTVICKAVSYLMGVSVSVSTLNLAAIALERYSAICRPLQARVWQTRSHAARVILATWLLSGLLMVPYPVYTVVQPVGPRILQCMHLWPSERVQQMWSVLLLILLFFIPGVVMAVAYGLISRELYLGLRFDGDNDSETQSRVRNQGGLPGGAAAPGPVHQNGGCRHVTSLTGEDSDGCYVQLPRSRLEMTTLTTPTTGPGPGPRPNQAKLLAKKRVVRMLLVIVLLFFVCWLPVYSANTWRAFDGPGARRALAGAPISFIHLLSYTSACANPLVYCFMHRRFRQACLDTCARCCPRPPRARPRPLPDEDPPTPSIASLSRLSYTTISTLGPG
Inhibitor
Name:
BDBM50005828
Synonyms:
2-(Adamantan-2-yloxycarbonylamino)-3-(1H-indol-3-yl)-2-methyl-thiopropionic acid S-phenethyl ester | CHEMBL288038
Type:
Small organic molecule
Emp. Form.:
C31H36N2O3S
Mol. Mass.:
516.694
SMILES:
CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)SCCc1ccccc1 |wU:19.20,17.29,wD:21.28,23.26,TLB:15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:16.17.25:22,(13.87,-8.6,;13.1,-7.26,;11.85,-6.36,;12.01,-4.82,;10.85,-3.82,;11.48,-2.41,;13,-2.54,;14.15,-1.52,;15.6,-1.97,;15.94,-3.5,;14.8,-4.53,;13.34,-4.05,;12.33,-8.6,;10.79,-8.58,;10.04,-7.23,;10.01,-9.89,;8.47,-9.86,;7.07,-9.28,;5.72,-9.76,;5.74,-11.24,;4.53,-12.51,;6.04,-12.09,;7.44,-12.65,;8.47,-11.39,;7.06,-11.72,;6.03,-10.5,;14.63,-7.15,;15.3,-5.77,;15.49,-8.41,;17.01,-8.32,;17.88,-9.59,;19.26,-8.86,;19.31,-7.35,;20.67,-6.62,;21.98,-7.45,;21.92,-8.99,;20.54,-9.7,)|