Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Angiotensin-converting enzyme
Ligand
BDBM50083382
Substrate
n/a
Meas. Tech.
ChEMBL_35381 (CHEMBL647297)
Ki
2200±n/a nM
Citation
 David, C; Bischoff, L; Meudal, H; Mothé, A; De Mota, N; DaNascimento, S; Llorens-Cortes, C; Fournié-Zaluski, MC; Roques, BP Investigation of subsite preferences in aminopeptidase A (EC 3.4.11.7) led to the design of the first highly potent and selective inhibitors of this enzyme. J Med Chem 42:5197-211 (2000) [PubMed] Article
David, C; Bischoff, L; Meudal, H; Mothé, A; De Mota, N; DaNascimento, S; Llorens-Cortes, C; Fournié-Zaluski, MC; Roques, BP Investigation of subsite preferences in aminopeptidase A (EC 3.4.11.7) led to the design of the first highly potent and selective inhibitors of this enzyme. J Med Chem 42:5197-211 (2000) [PubMed] Article More Info.:
Target
Name:
Angiotensin-converting enzyme
Synonyms:
ACE_RAT | Ace | Angiotensin-converting enzyme | Dcp1
Type:
PROTEIN
Mol. Mass.:
150907.81
Organism:
Rattus norvegicus
Description:
ChEMBL_35219
Residue:
1313
Sequence:
MGAASGQRGRWPLSPPLLMLSLLLLLLLPPSPAPALDPGLQPGNFSADEAGAQLFADSYNSSAEVVMFQSTAASWAHDTNITEENARLQEEAALINQEFAEVWGKKAKELYESIWQNFTDQKLRRIIGSVQTLGPANLPLTQRLQYNSLLSNMSRIYSTGKVCFPNKTATCWSLDPELTNILASSRNYAKVLFAWEGWHDAVGIPLRPLYQDFTALSNEAYRQDGFSDTGAYWRSWYESPSFEESLEHLYHQVEPLYLNLHAFVRRALHRRYGDKYINLRGPIPAHLLGDMWAQSWENIYDMVVPFPDKPNLDVTSTMVQKGWNATHMFRVAEEFFTSLGLSPMPPEFWAESMLEKPADGREVVCHASAWDFYNRKDFRIKQCTRVTMDQLSTVHHEMGHVQYYLQYKDLHVSLRRGANPGFHEAIGDVLALSVSTPAHLHKIGLLDRVANDIESDINYLLKMALEKIAFLPFGYLVDQWRWGVFSGRTPPSRYNYDWWYLRTKYQGICPPVARNETHFDAGAKFHIPSVTPYIRYFVSFVLQFQFHQALCKEAGHQGPLHQCDIYQSTKAGAKLQQVLQAGCSRPWQEVLKDLVGSDALDASALMEYFQPVSQWLQEQNQRNGEVLGWPEYQWRPPLPDNYPEGIDLETDEAKANRFVEEYDRTAKVLWNEYAEANWHYNTNITIEGSKILLQKNKEVSNHTLKYGTWAKTFDVSNFQNSTIKRIIKKVQNVDRAVLPPNELEEYNQILLDMETTYSVANVCYTNGTCLSLEPDLTNIMATSRKYEELLWVWKSWRDKVGRAILPFFPKYVDFSNKIAKLNGYSDAGDSWRSSYESDDLEQDLEKLYQELQPLYLNLHAYVRRSLHRHYGSEYINLDGPIPAHLLGNMWAQTWSNIYDLVAPFPSAPSIDATEAMIKQGWTPRRIFKEADNFFTSLGLLPVPPEFWNKSMLEKPTDGREVVCHASAWDFYNGKDFRIKQCTSVNMEELVIAHHEMGHIQYFMQYKDLPVTFREGANPGFHEAIGDVLALSVSTPKHLHSLNLLSSEGSGYEHDINFLMKMALDKIAFIPFSYLIDQWRWRVFDGSITKENYNQEWWSLRLKYQGLCPPVPRSQGDFDPGSKFHVPANVPYIRYFISFIIQFQFHEALCRAAGHTGPLYKCDIYQSKEAGKLLADAMKLGYSKQWPEAMKIITGQPNMSASAIMNYFKPLTEWLVTENRRHGETLGWPEYTWTPNTARAEGSLPESSRVNFLGMYLEPQQARVGQWVLLFLGVALLVATVGLAHRLYNIHNHHSLRRPHRGPQFGSEVELRHS
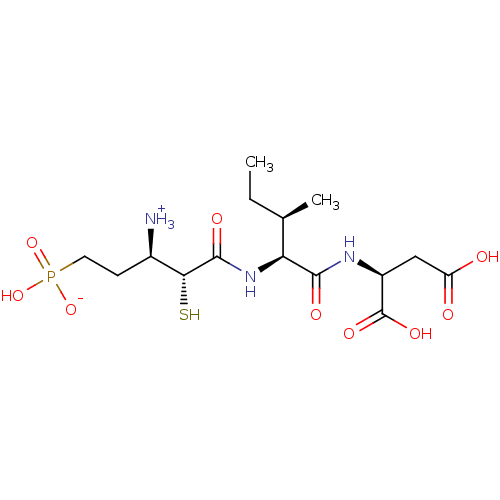
Inhibitor
Name:
BDBM50083382
Synonyms:
1-{[1-(1,2-Dicarboxy-ethylcarbamoyl)-2-methyl-butylcarbamoyl]-mercapto-methyl}-3-phosphono-propyl-ammonium | CHEMBL356858
Type:
Small organic molecule
Emp. Form.:
C15H28N3O9PS
Mol. Mass.:
457.436
SMILES:
CC[C@@H](C)[C@H](NC(=O)[C@H](S)[C@H]([NH3+])CCP(O)([O-])=O)C(=O)N[C@@H](CC(O)=O)C(O)=O