Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Solute carrier family 15 member 2
Ligand
BDBM50335523
Substrate
n/a
Meas. Tech.
ChEMBL_762551 (CHEMBL1817008)
Ki
20000000±n/a nM
Citation
 Pedretti, A; De Luca, L; Marconi, C; Regazzoni, L; Aldini, G; Vistoli, G Fragmental modeling of hPepT2 and analysis of its binding features by docking studies and pharmacophore mapping. Bioorg Med Chem 19:4544-51 (2011) [PubMed] Article
Pedretti, A; De Luca, L; Marconi, C; Regazzoni, L; Aldini, G; Vistoli, G Fragmental modeling of hPepT2 and analysis of its binding features by docking studies and pharmacophore mapping. Bioorg Med Chem 19:4544-51 (2011) [PubMed] Article More Info.:
Target
Name:
Solute carrier family 15 member 2
Synonyms:
Kidney H(+)/peptide cotransporter | Oligopeptide transporter, kidney isoform | PEPT2 | Peptide transporter 2 | S15A2_HUMAN | SLC15A2
Type:
PROTEIN
Mol. Mass.:
81795.62
Organism:
Homo sapiens (Human)
Description:
ChEMBL_762551
Residue:
729
Sequence:
MNPFQKNESKETLFSPVSIEEVPPRPPSPPKKPSPTICGSNYPLSIAFIVVNEFCERFSYYGMKAVLILYFLYFLHWNEDTSTSIYHAFSSLCYFTPILGAAIADSWLGKFKTIIYLSLVYVLGHVIKSLGALPILGGQVVHTVLSLIGLSLIALGTGGIKPCVAAFGGDQFEEKHAEERTRYFSVFYLSINAGSLISTFITPMLRGDVQCFGEDCYALAFGVPGLLMVIALVVFAMGSKIYNKPPPEGNIVAQVFKCIWFAISNRFKNRSGDIPKRQHWLDWAAEKYPKQLIMDVKALTRVLFLYIPLPMFWALLDQQGSRWTLQAIRMNRNLGFFVLQPDQMQVLNPLLVLIFIPLFDFVIYRLVSKCGINFSSLRKMAVGMILACLAFAVAAAVEIKINEMAPAQPGPQEVFLQVLNLADDEVKVTVVGNENNSLLIESIKSFQKTPHYSKLHLKTKSQDFHFHLKYHNLSLYTEHSVQEKNWYSLVIREDGNSISSMMVKDTESRTTNGMTTVRFVNTLHKDVNISLSTDTSLNVGEDYGVSAYRTVQRGEYPAVHCRTEDKNFSLNLGLLDFGAAYLFVITNNTNQGLQAWKIEDIPANKMSIAWQLPQYALVTAGEVMFSVTGLEFSYSQAPSSMKSVLQAAWLLTIAVGNIIVLVVAQFSGLVQWAEFILFSCLLLVICLIFSIMGYYYVPVKTEDMRGPADKHIPHIQGNMIKLETKKTKL
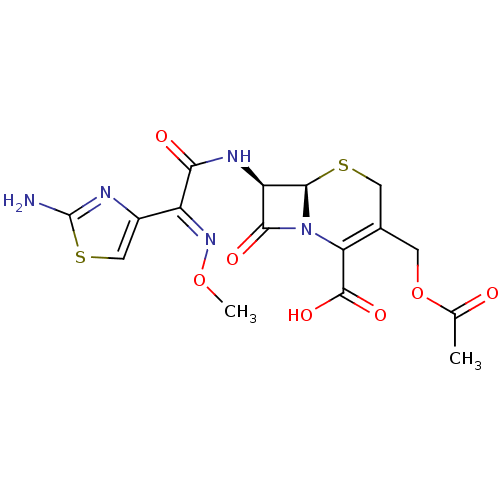
Inhibitor
Name:
BDBM50335523
Synonyms:
(6R,7R)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid | (6R,7R)-3-Acetoxymethyl-7-[2-(2-amino-thiazol-4-yl)-2-hydroxyimino-acetylamino]-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid | (6R,7R)-3-Acetoxymethyl-7-{2-(2-amino-thiazol-4-yl)-2-[(E)-methoxyimino]-acetylamino}-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid | (6R,7R)-3-Acetoxymethyl-7-{2-(2-amino-thiazol-4-yl)-2-[(Z)-methoxyimino]-acetylamino}-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid | (6R,7R,E)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid | (6R,7R,Z)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid | (6R,7R,Z)-3-(acetoxymethyl)-7-(2-(5-aminothiophen-3-yl)-2-(methoxyimino)acetamido)-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid | (6R,7S,Z)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid | (6S,7S,E)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid | (Cefotaxime)3-Acetoxymethyl-7-[2-(2-amino-thiazol-4-yl)-2-methoxyimino-acetylamino]-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid | (R)-3-Acetoxymethyl-7-{2-(2-amino-thiazol-4-yl)-2-[(E)-methoxyimino]-acetylamino}-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid | 3-Acetoxymethyl-7-[2-(2-amino-thiazol-4-yl)-2-methoxyimino-acetylamino]-5,8-dioxo-5lambda*4*-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid | 3-Acetoxymethyl-7-[2-(2-amino-thiazol-4-yl)-2-methoxyimino-acetylamino]-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid | 3-Acetoxymethyl-7-[2-(2-amino-thiazol-4-yl)-2-methoxyimino-acetylamino]-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid (Cefotaxime) | 3-Acetoxymethyl-7-[2-(2-amino-thiazol-4-yl)-2-methoxyimino-acetylamino]-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid anion | 3-Acetoxymethyl-7-[2-(2-amino-thiazol-4-yl)-2-methoxyimino-acetylamino]-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid(Cefotaxime) | 3-Acetoxymethyl-7-{2-(2-amino-thiazol-4-yl)-2-[(Z)-methoxyimino]-acetylamino}-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid anion | CHEMBL102 | Cefotax | Claforan | Kefotex | cefotaxim | cefotaxime
Type:
Small organic molecule
Emp. Form.:
C16H17N5O7S2
Mol. Mass.:
455.465
SMILES:
CO\N=C(\C(=O)N[C@H]1[C@H]2SCC(COC(C)=O)=C(N2C1=O)C(O)=O)c1csc(N)n1 |r,c:16|