Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 2C9
Ligand
BDBM50380679
Substrate
n/a
Meas. Tech.
ChEMBL_813215 (CHEMBL2020947)
IC50
9800±n/a nM
Citation
 Mirguet, O; Lamotte, Y; Donche, F; Toum, J; Gellibert, F; Bouillot, A; Gosmini, R; Nguyen, VL; Delannée, D; Seal, J; Blandel, F; Boullay, AB; Boursier, E; Martin, S; Brusq, JM; Krysa, G; Riou, A; Tellier, R; Costaz, A; Huet, P; Dudit, Y; Trottet, L; Kirilovsky, J; Nicodeme, E From ApoA1 upregulation to BET family bromodomain inhibition: discovery of I-BET151. Bioorg Med Chem Lett 22:2963-7 (2012) [PubMed] Article
Mirguet, O; Lamotte, Y; Donche, F; Toum, J; Gellibert, F; Bouillot, A; Gosmini, R; Nguyen, VL; Delannée, D; Seal, J; Blandel, F; Boullay, AB; Boursier, E; Martin, S; Brusq, JM; Krysa, G; Riou, A; Tellier, R; Costaz, A; Huet, P; Dudit, Y; Trottet, L; Kirilovsky, J; Nicodeme, E From ApoA1 upregulation to BET family bromodomain inhibition: discovery of I-BET151. Bioorg Med Chem Lett 22:2963-7 (2012) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 2C9
Synonyms:
(R)-limonene 6-monooxygenase | (S)-limonene 6-monooxygenase | CP2C9_HUMAN | CYP2C10 | CYP2C9 | CYPIIC9 | Cytochrome P450 2C9 (CYP2C9 ) | Cytochrome P450 2C9 (CYP2C9) | P-450MP | P450 MP-4/MP-8 | P450 PB-1 | S-mephenytoin 4-hydroxylase
Type:
Enzyme
Mol. Mass.:
55636.33
Organism:
Homo sapiens (Human)
Description:
P11712
Residue:
490
Sequence:
MDSLVVLVLCLSCLLLLSLWRQSSGRGKLPPGPTPLPVIGNILQIGIKDISKSLTNLSKVYGPVFTLYFGLKPIVVLHGYEAVKEALIDLGEEFSGRGIFPLAERANRGFGIVFSNGKKWKEIRRFSLMTLRNFGMGKRSIEDRVQEEARCLVEELRKTKASPCDPTFILGCAPCNVICSIIFHKRFDYKDQQFLNLMEKLNENIKILSSPWIQICNNFSPIIDYFPGTHNKLLKNVAFMKSYILEKVKEHQESMDMNNPQDFIDCFLMKMEKEKHNQPSEFTIESLENTAVDLFGAGTETTSTTLRYALLLLLKHPEVTAKVQEEIERVIGRNRSPCMQDRSHMPYTDAVVHEVQRYIDLLPTSLPHAVTCDIKFRNYLIPKGTTILISLTSVLHDNKEFPNPEMFDPHHFLDEGGNFKKSKYFMPFSAGKRICVGEALAGMELFLFLTSILQNFNLKSLVDPKNLDTTPVVNGFASVPPFYQLCFIPV
Inhibitor
Name:
BDBM50380679
Synonyms:
CHEMBL2017286
Type:
Small organic molecule
Emp. Form.:
C22H19N5O3
Mol. Mass.:
401.418
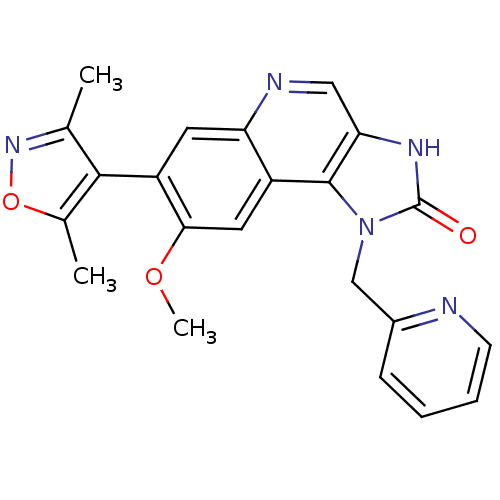
SMILES:
COc1cc2c3n(Cc4ccccn4)c(=O)[nH]c3cnc2cc1-c1c(C)noc1C |(44.75,-24.87,;44.75,-26.41,;46.08,-27.18,;47.41,-26.4,;48.74,-27.17,;50.07,-26.4,;50.38,-24.89,;49.61,-23.56,;50.38,-22.23,;49.6,-20.9,;50.36,-19.57,;51.91,-19.57,;52.68,-20.9,;51.91,-22.23,;51.9,-24.72,;52.66,-23.38,;52.54,-26.11,;51.41,-27.15,;51.42,-28.71,;50.08,-29.48,;48.75,-28.71,;47.41,-29.49,;46.08,-28.72,;44.74,-29.49,;44.71,-31.03,;45.94,-31.96,;43.24,-31.48,;42.36,-30.21,;43.29,-28.99,;42.84,-27.51,)|