Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Rho-associated protein kinase 2
Ligand
BDBM50311409
Substrate
n/a
Meas. Tech.
ChEMBL_816474 (CHEMBL2024961)
IC50
2.8±n/a nM
Citation
 Lavogina, D; Kalind, K; Bredihhina, J; Hurt, M; Vaasa, A; Kasari, M; Enkvist, E; Raidaru, G; Uri, A Conjugates of 5-isoquinolinesulfonylamides and oligo-D-arginine possess high affinity and selectivity towards Rho kinase (ROCK). Bioorg Med Chem Lett 22:3425-30 (2012) [PubMed] Article
Lavogina, D; Kalind, K; Bredihhina, J; Hurt, M; Vaasa, A; Kasari, M; Enkvist, E; Raidaru, G; Uri, A Conjugates of 5-isoquinolinesulfonylamides and oligo-D-arginine possess high affinity and selectivity towards Rho kinase (ROCK). Bioorg Med Chem Lett 22:3425-30 (2012) [PubMed] Article More Info.:
Target
Name:
Rho-associated protein kinase 2
Synonyms:
KIAA0619 | ROCK-II | ROCK2 | ROCK2_HUMAN | Rho kinase 2 (ROCKII) | Rho-associated protein kinase 2 (ROCK-2) | Rho-associated protein kinase 2 (ROCK2) | Rho-associated protein kinase 2 (ROCKII) | Rho-associated protein kinase 2/Transforming protein RhoA | Rho-associated, coiled-coil-containing protein kinase 2 | Rho-associated, coiled-coil-containing protein kinase II | Serine/threonine-protein kinase RIO2 | p164 ROCK-2
Type:
Protein
Mol. Mass.:
160885.43
Organism:
Homo sapiens (Human)
Description:
O75116
Residue:
1388
Sequence:
MSRPPPTGKMPGAPETAPGDGAGASRQRKLEALIRDPRSPINVESLLDGLNSLVLDLDFPALRKNKNIDNFLNRYEKIVKKIRGLQMKAEDYDVVKVIGRGAFGEVQLVRHKASQKVYAMKLLSKFEMIKRSDSAFFWEERDIMAFANSPWVVQLFYAFQDDRYLYMVMEYMPGGDLVNLMSNYDVPEKWAKFYTAEVVLALDAIHSMGLIHRDVKPDNMLLDKHGHLKLADFGTCMKMDETGMVHCDTAVGTPDYISPEVLKSQGGDGFYGRECDWWSVGVFLYEMLVGDTPFYADSLVGTYSKIMDHKNSLCFPEDAEISKHAKNLICAFLTDREVRLGRNGVEEIRQHPFFKNDQWHWDNIRETAAPVVPELSSDIDSSNFDDIEDDKGDVETFPIPKAFVGNQLPFIGFTYYRENLLLSDSPSCRETDSIQSRKNEESQEIQKKLYTLEEHLSNEMQAKEELEQKCKSVNTRLEKTAKELEEEITLRKSVESALRQLEREKALLQHKNAEYQRKADHEADKKRNLENDVNSLKDQLEDLKKRNQNSQISTEKVNQLQRQLDETNALLRTESDTAARLRKTQAESSKQIQQLESNNRDLQDKNCLLETAKLKLEKEFINLQSALESERRDRTHGSEIINDLQGRICGLEEDLKNGKILLAKVELEKRQLQERFTDLEKEKSNMEIDMTYQLKVIQQSLEQEEAEHKATKARLADKNKIYESIEEAKSEAMKEMEKKLLEERTLKQKVENLLLEAEKRCSLLDCDLKQSQQKINELLKQKDVLNEDVRNLTLKIEQETQKRCLTQNDLKMQTQQVNTLKMSEKQLKQENNHLMEMKMNLEKQNAELRKERQDADGQMKELQDQLEAEQYFSTLYKTQVRELKEECEEKTKLGKELQQKKQELQDERDSLAAQLEITLTKADSEQLARSIAEEQYSDLEKEKIMKELEIKEMMARHKQELTEKDATIASLEETNRTLTSDVANLANEKEELNNKLKDVQEQLSRLKDEEISAAAIKAQFEKQLLTERTLKTQAVNKLAEIMNRKEPVKRGNDTDVRRKEKENRKLHMELKSEREKLTQQMIKYQKELNEMQAQIAEESQIRIELQMTLDSKDSDIEQLRSQLQALHIGLDSSSIGSGPGDAEADDGFPESRLEGWLSLPVRNNTKKFGWVKKYVIVSSKKILFYDSEQDKEQSNPYMVLDIDKLFHVRPVTQTDVYRADAKEIPRIFQILYANEGESKKEQEFPVEPVGEKSNYICHKGHEFIPTLYHFPTNCEACMKPLWHMFKPPPALECRRCHIKCHKDHMDKKEEIIAPCKVYYDISTAKNLLLLANSTEEQQKWVSRLVKKIPKKPPAPDPFARSSPRTSMKIQQNQSIRRPSRQLAPNKPS
Inhibitor
Name:
BDBM50311409
Synonyms:
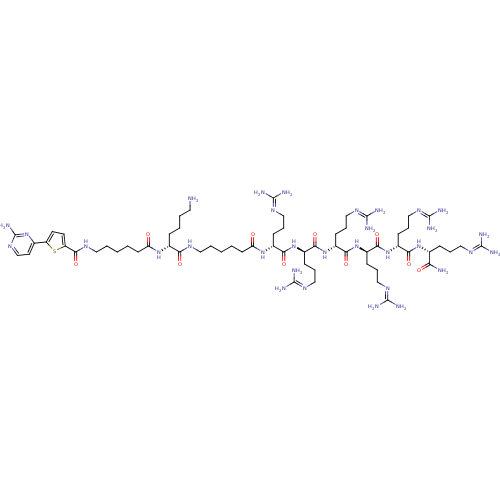
CHEMBL1077375 | N-((6R,9R,12R,15R,18R,21R,31R)-1-amino-31-(4-aminobutyl)-6-carbamoyl-9,12,15,18,21-pentakis(3-guanidinopropyl)-1-imino-8,11,14,17,20,23,30,33-octaoxo-2,7,10,13,16,19,22,29,32-nonaazaoctatriacontan-38-yl)-5-(2-aminopyrimidin-4-yl)thiophene-2-carboxamide
Type:
Small organic molecule
Emp. Form.:
C63H114N32O10S
Mol. Mass.:
1511.853
SMILES:
[#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c1ccc(s1)-c1ccnc(-[#7])n1)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O |r|