Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
E3 ubiquitin-protein ligase XIAP
Ligand
BDBM44276
Substrate
n/a
Meas. Tech.
ChEMBL_971509 (CHEMBL2406571)
Ki
390±n/a nM
Citation
 Ardecky, RJ; Welsh, K; Finlay, D; Lee, PS; González-López, M; Ganji, SR; Ravanan, P; Mace, PD; Riedl, SJ; Vuori, K; Reed, JC; Cosford, ND Design, synthesis and evaluation of inhibitor of apoptosis protein (IAP) antagonists that are highly selective for the BIR2 domain of XIAP. Bioorg Med Chem Lett 23:4253-7 (2013) [PubMed] Article
Ardecky, RJ; Welsh, K; Finlay, D; Lee, PS; González-López, M; Ganji, SR; Ravanan, P; Mace, PD; Riedl, SJ; Vuori, K; Reed, JC; Cosford, ND Design, synthesis and evaluation of inhibitor of apoptosis protein (IAP) antagonists that are highly selective for the BIR2 domain of XIAP. Bioorg Med Chem Lett 23:4253-7 (2013) [PubMed] Article More Info.:
Target
Name:
E3 ubiquitin-protein ligase XIAP
Synonyms:
API3 | BIRC4 | E3 ubiquitin-protein ligase XIAP | IAP3 | Inhibitor of apoptosis protein 3 | Inhibitor of apoptosis protein 3 (XIAP) | X-linked inhibitor of apoptosis | X-linked inhibitor of apoptosis protein (XIAP) | XIAP | XIAP_HUMAN
Type:
Protein
Mol. Mass.:
56685.27
Organism:
Homo sapiens (Human)
Description:
P98170
Residue:
497
Sequence:
MTFNSFEGSKTCVPADINKEEEFVEEFNRLKTFANFPSGSPVSASTLARAGFLYTGEGDTVRCFSCHAAVDRWQYGDSAVGRHRKVSPNCRFINGFYLENSATQSTNSGIQNGQYKVENYLGSRDHFALDRPSETHADYLLRTGQVVDISDTIYPRNPAMYSEEARLKSFQNWPDYAHLTPRELASAGLYYTGIGDQVQCFCCGGKLKNWEPCDRAWSEHRRHFPNCFFVLGRNLNIRSESDAVSSDRNFPNSTNLPRNPSMADYEARIFTFGTWIYSVNKEQLARAGFYALGEGDKVKCFHCGGGLTDWKPSEDPWEQHAKWYPGCKYLLEQKGQEYINNIHLTHSLEECLVRTTEKTPSLTRRIDDTIFQNPMVQEAIRMGFSFKDIKKIMEEKIQISGSNYKSLEVLVADLVNAQKDSMQDESSQTSLQKEISTEEQLRRLQEEKLCKICMDRNIAIVFVPCGHLVTCKQCAEAVDKCPMCYTVITFKQKIFMS
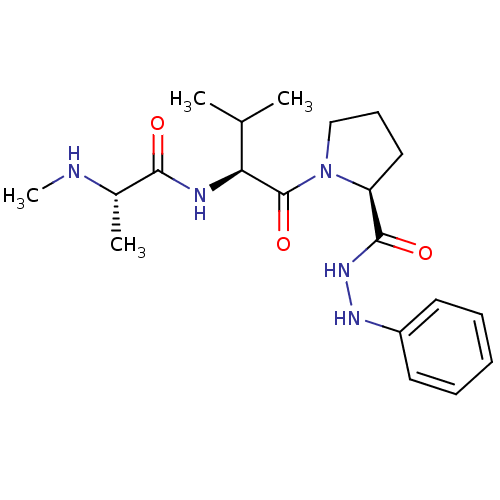
Inhibitor
Name:
BDBM44276
Synonyms:
(2S)-N-[(1S)-1-[(2S)-2-(anilinocarbamoyl)pyrrolidine-1-carbonyl]-2-methyl-propyl]-2-(methylamino)propionamide;formic acid | (2S)-N-[(2S)-1-[(2S)-2-(anilinocarbamoyl)pyrrolidin-1-yl]-3-methyl-1-oxobutan-2-yl]-2-(methylamino)propanamide;formic acid | CHEMBL1625066 | MLS-0391011.0001 | cid_25241673 | formic acid;(2S)-2-(methylamino)-N-[(2S)-3-methyl-1-oxo-1-[(2S)-2-[oxo-(phenylhydrazo)methyl]-1-pyrrolidinyl]butan-2-yl]propanamide | methanoic acid;(2S)-2-(methylamino)-N-[(2S)-3-methyl-1-oxidanylidene-1-[(2S)-2-(phenylazanylcarbamoyl)pyrrolidin-1-yl]butan-2-yl]propanamide
Type:
Small organic molecule
Emp. Form.:
C20H31N5O3
Mol. Mass.:
389.4918
SMILES:
CN[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)NNc1ccccc1