Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Suppressor of tumorigenicity 14 protein
Ligand
BDBM50017932
Substrate
n/a
Meas. Tech.
ChEMBL_1364433 (CHEMBL3294545)
Ki
1131±n/a nM
Citation
 Goswami, R; Mukherjee, S; Ghadiyaram, C; Wohlfahrt, G; Sistla, RK; Nagaraj, J; Satyam, LK; Subbarao, K; Palakurthy, RK; Gopinath, S; Krishnamurthy, NR; Ikonen, T; Moilanen, A; Subramanya, HS; Kallio, P; Ramachandra, M Structure-guided discovery of 1,3,5 tri-substituted benzenes as potent and selective matriptase inhibitors exhibiting in vivo antitumor efficacy. Bioorg Med Chem 22:3187-203 (2014) [PubMed] Article
Goswami, R; Mukherjee, S; Ghadiyaram, C; Wohlfahrt, G; Sistla, RK; Nagaraj, J; Satyam, LK; Subbarao, K; Palakurthy, RK; Gopinath, S; Krishnamurthy, NR; Ikonen, T; Moilanen, A; Subramanya, HS; Kallio, P; Ramachandra, M Structure-guided discovery of 1,3,5 tri-substituted benzenes as potent and selective matriptase inhibitors exhibiting in vivo antitumor efficacy. Bioorg Med Chem 22:3187-203 (2014) [PubMed] Article More Info.:
Target
Name:
Suppressor of tumorigenicity 14 protein
Synonyms:
Epithin | Hepatocyte growth factor activator/Serine protease hepsin/Suppressor of tumorigenicity 14 protein | MT-SP1 | Membrane-type serine protease 1 | PRSS14 | Prostamin | SNC19 | ST14 | ST14_HUMAN | Serine protease TADG-15 | Suppressor of tumorigenicity 14 protein | Suppressor of tumorigenicity protein 14 | TADG15
Type:
Single-pass type II membrane protein
Mol. Mass.:
94769.23
Organism:
Homo sapiens (Human)
Description:
Q9Y5Y6
Residue:
855
Sequence:
MGSDRARKGGGGPKDFGAGLKYNSRHEKVNGLEEGVEFLPVNNVKKVEKHGPGRWVVLAAVLIGLLLVLLGIGFLVWHLQYRDVRVQKVFNGYMRITNENFVDAYENSNSTEFVSLASKVKDALKLLYSGVPFLGPYHKESAVTAFSEGSVIAYYWSEFSIPQHLVEEAERVMAEERVVMLPPRARSLKSFVVTSVVAFPTDSKTVQRTQDNSCSFGLHARGVELMRFTTPGFPDSPYPAHARCQWALRGDADSVLSLTFRSFDLASCDERGSDLVTVYNTLSPMEPHALVQLCGTYPPSYNLTFHSSQNVLLITLITNTERRHPGFEATFFQLPRMSSCGGRLRKAQGTFNSPYYPGHYPPNIDCTWNIEVPNNQHVKVRFKFFYLLEPGVPAGTCPKDYVEINGEKYCGERSQFVVTSNSNKITVRFHSDQSYTDTGFLAEYLSYDSSDPCPGQFTCRTGRCIRKELRCDGWADCTDHSDELNCSCDAGHQFTCKNKFCKPLFWVCDSVNDCGDNSDEQGCSCPAQTFRCSNGKCLSKSQQCNGKDDCGDGSDEASCPKVNVVTCTKHTYRCLNGLCLSKGNPECDGKEDCSDGSDEKDCDCGLRSFTRQARVVGGTDADEGEWPWQVSLHALGQGHICGASLISPNWLVSAAHCYIDDRGFRYSDPTQWTAFLGLHDQSQRSAPGVQERRLKRIISHPFFNDFTFDYDIALLELEKPAEYSSMVRPICLPDASHVFPAGKAIWVTGWGHTQYGGTGALILQKGEIRVINQTTCENLLPQQITPRMMCVGFLSGGVDSCQGDSGGPLSSVEADGRIFQAGVVSWGDGCAQRNKPGVYTRLPLFRDWIKENTGV
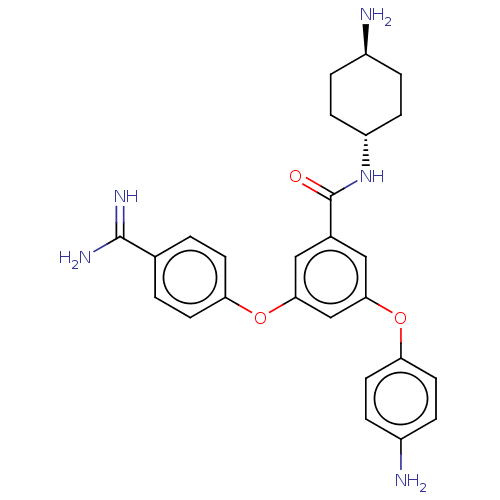
Inhibitor
Name:
BDBM50017932
Synonyms:
CHEMBL3289039
Type:
Small organic molecule
Emp. Form.:
C26H29N5O3
Mol. Mass.:
459.5402
SMILES:
N[C@H]1CC[C@@H](CC1)NC(=O)c1cc(Oc2ccc(N)cc2)cc(Oc2ccc(cc2)C(N)=N)c1 |r,wU:1.0,wD:4.7,(18.33,-12.11,;17,-11.34,;15.67,-12.11,;14.33,-11.34,;14.33,-9.8,;15.67,-9.03,;17,-9.8,;13,-9.03,;11.66,-9.8,;11.66,-11.34,;10.33,-9.03,;9,-9.8,;7.66,-9.03,;6.33,-9.8,;5,-9.03,;3.66,-9.8,;2.33,-9.03,;2.33,-7.49,;.99,-6.72,;3.66,-6.72,;5,-7.49,;7.66,-7.49,;9,-6.72,;9,-5.18,;10.33,-4.41,;10.33,-2.87,;11.66,-2.1,;13,-2.87,;13,-4.41,;11.66,-5.18,;14.33,-2.1,;15.67,-2.87,;14.33,-.56,;10.33,-7.49,)|