Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Potassium voltage-gated channel subfamily H member 2
Ligand
BDBM4814
Substrate
n/a
Meas. Tech.
ChEMBL_1365396 (CHEMBL3296755)
IC50
500±n/a nM
Citation
More Info.:
Target
Name:
Potassium voltage-gated channel subfamily H member 2
Synonyms:
1,3-beta-glucan synthase component GLS2 | Cytochrome P450 3A4 | ERG | ERG1 | Eag-related protein 1 | Ether a-go-go related gene potassium channel (hERG) | Ether-a-go-go-related gene (HERG) | Ether-a-go-go-related gene potassium channel (hERG) | Ether-a-go-go-related gene potassium channel 1 | Ether-a-go-go-related gene potassium channel 1 (HERG) | Ether-a-go-go-related gene potassium channel 1 (hERG1) | Ether-a-go-go-related protein (hERG) | Ether-a-go-go-related protein 1 | Ether-a-go-go-related protein 1 (HERG) | H-ERG | HERG | KCNH2 | KCNH2_HUMAN | Potassium voltage-gated channel subfamily H member 2 (hERG) | Transcriptional regulator ERG | Voltage-gated potassium channel subunit Kv11.1 | eag homolog | hERG Potassium Channel 1 | putative potassium channel subunit
Type:
Multi-pass membrane protein
Mol. Mass.:
126672.65
Organism:
Homo sapiens (Human)
Description:
Q12809
Residue:
1159
Sequence:
MPVRRGHVAPQNTFLDTIIRKFEGQSRKFIIANARVENCAVIYCNDGFCELCGYSRAEVMQRPCTCDFLHGPRTQRRAAAQIAQALLGAEERKVEIAFYRKDGSCFLCLVDVVPVKNEDGAVIMFILNFEVVMEKDMVGSPAHDTNHRGPPTSWLAPGRAKTFRLKLPALLALTARESSVRSGGAGGAGAPGAVVVDVDLTPAAPSSESLALDEVTAMDNHVAGLGPAEERRALVGPGSPPRSAPGQLPSPRAHSLNPDASGSSCSLARTRSRESCASVRRASSADDIEAMRAGVLPPPPRHASTGAMHPLRSGLLNSTSDSDLVRYRTISKIPQITLNFVDLKGDPFLASPTSDREIIAPKIKERTHNVTEKVTQVLSLGADVLPEYKLQAPRIHRWTILHYSPFKAVWDWLILLLVIYTAVFTPYSAAFLLKETEEGPPATECGYACQPLAVVDLIVDIMFIVDILINFRTTYVNANEEVVSHPGRIAVHYFKGWFLIDMVAAIPFDLLIFGSGSEELIGLLKTARLLRLVRVARKLDRYSEYGAAVLFLLMCTFALIAHWLACIWYAIGNMEQPHMDSRIGWLHNLGDQIGKPYNSSGLGGPSIKDKYVTALYFTFSSLTSVGFGNVSPNTNSEKIFSICVMLIGSLMYASIFGNVSAIIQRLYSGTARYHTQMLRVREFIRFHQIPNPLRQRLEEYFQHAWSYTNGIDMNAVLKGFPECLQADICLHLNRSLLQHCKPFRGATKGCLRALAMKFKTTHAPPGDTLVHAGDLLTALYFISRGSIEILRGDVVVAILGKNDIFGEPLNLYARPGKSNGDVRALTYCDLHKIHRDDLLEVLDMYPEFSDHFWSSLEITFNLRDTNMIPGSPGSTELEGGFSRQRKRKLSFRRRTDKDTEQPGEVSALGPGRAGAGPSSRGRPGGPWGESPSSGPSSPESSEDEGPGRSSSPLRLVPFSSPRPPGEPPGGEPLMEDCEKSSDTCNPLSGAFSGVSNIFSFWGDSRGRQYQELPRCPAPTPSLLNIPLSSPGRRPRGDVESRLDALQRQLNRLETRLSADMATVLQLLQRQMTLVPPAYSAVTTPGPGPTSTSPLLPVSPLPTLTLDSLSQVSQFMACEELPPGAPELPQEGPTRRLSLPGQLGALTSQPLHRHGSDPGS
Inhibitor
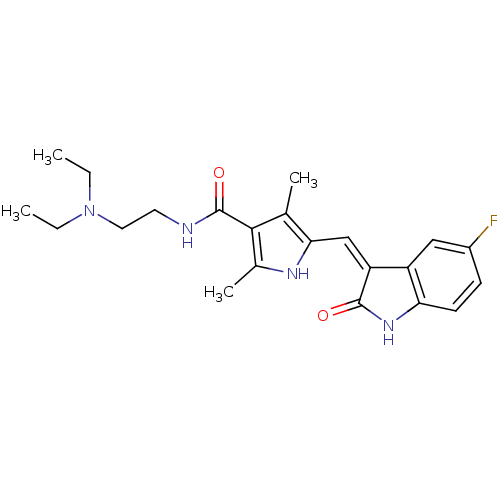
Name:
BDBM4814
Synonyms:
CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide | N-[2-(diethylamino)ethyl]-5-{[(3Z)-5-fluoro-2-oxo-2,3-dihydro-1H-indol-3-ylidene]methyl}-2,4-dimethyl-1H-pyrrole-3-carboxamide | SU11248 | SUNITINIB | SUNITINIB MALATE | US10464902, Sunitinib | US9163010, Sunitinib | US9914707, SU11248
Type:
Small organic molecule
Emp. Form.:
C22H27FN4O2
Mol. Mass.:
398.4738
SMILES:
CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C