Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Sucrase-isomaltase, intestinal
Ligand
BDBM50242271
Substrate
n/a
Meas. Tech.
ChEMBL_1454616 (CHEMBL3366728)
IC50
1000±n/a nM
Citation
 Natori, Y; Sakuma, T; Yoshimura, Y; Kinami, K; Hirokami, Y; Sato, K; Adachi, I; Kato, A; Takahata, H Synthesis and biological evaluation ofa-1-C-4'-arylbutyl-L-arabinoiminofuranoses, a new class ofa-glucosidase inhibitors. Bioorg Med Chem Lett 24:3298-301 (2014) [PubMed] Article
Natori, Y; Sakuma, T; Yoshimura, Y; Kinami, K; Hirokami, Y; Sato, K; Adachi, I; Kato, A; Takahata, H Synthesis and biological evaluation ofa-1-C-4'-arylbutyl-L-arabinoiminofuranoses, a new class ofa-glucosidase inhibitors. Bioorg Med Chem Lett 24:3298-301 (2014) [PubMed] Article More Info.:
Target
Name:
Sucrase-isomaltase, intestinal
Synonyms:
SUIS_RAT | Si | Sucrase-isomaltase | alpha-Glucosidase (α-Glucosidase)
Type:
Enzyme
Mol. Mass.:
210329.04
Organism:
Rattus norvegicus (Rat)
Description:
P23739
Residue:
1841
Sequence:
MAKKKFSALEISLIVLFIIVTAIAIALVTVLATKVPAVEEIKSPTPTSNSTPTSTPTSTSTPTSTSTPSPGKCPPEQGEPINERINCIPEQHPTKAICEERGCCWRPWNNTVIPWCFFADNHGYNAESITNENAGLKATLNRIPSPTLFGEDIKSVILTTQTQTGNRFRFKITDPNNKRYEVPHQFVKEETGIPAADTLYDVQVSENPFSIKVIRKSNNKVLCDTSVGPLLYSNQYLQISTRLPSEYIYGFGGHIHKRFRHDLYWKTWPIFTRDEIPGDNNHNLYGHQTFFMGIGDTSGKSYGVFLMNSNAMEVFIQPTPIITYRVTGGILDFYIFLGDTPEQVVQQYQEVHWRPAMPAYWNLGFQLSRWNYGSLDTVSEVVRRNREAGIPYDAQVTDIDYMEDHKEFTYDRVKFNGLPEFAQDLHNHGKYIIILDPAISINKRANGAEYQTYVRGNEKNVWVNESDGTTPLIGEVWPGLTVYPDFTNPQTIEWWANECNLFHQQVEYDGLWIDMNEVSSFIQGSLNLKGVLLIVLNYPPFTPGILDKVMYSKTLCMDAVQHWGKQYDVHSLYGYSMAIATEQAVERVFPNKRSFILTRSTFGGSGRHANHWLGDNTASWEQMEWSITGMLEFGIFGMPLVGATSCGFLADTTEELCRRWMQLGAFYPFSRNHNAEGYMEQDPAYFGQDSSRHYLTIRYTLLPFLYTLFYRAHMFGETVARPFLYEFYDDTNSWIEDTQFLWGPALLITPVLRPGVENVSAYIPNATWYDYETGIKRPWRKERINMYLPGDKIGLHLRGGYIIPTQEPDVTTTASRKNPLGLIVALDDNQAAKGELFWDDGESKDSIEKKMYILYTFSVSNNELVLNCTHSSYAEGTSLAFKTIKVLGLREDVRSITVGENDQQMATHTNFTFDSANKILSITALNFNLAGSFIVRWCRTFSDNEKFTCYPDVGTATEGTCTQRGCLWQPVSGLSNVPPYYFPPENNPYTLTSIQPLPTGITAELQLNPPNARIKLPSNPISTLRVGVKYHPNDMLQFKIYDAQHKRYEVPVPLNIPDTPTSSNERLYDVEIKENPFGIQVRRRSSGKLIWDSRLPGFGFNDQFIQISTRLPSNYLYGFGEVEHTAFKRDLNWHTWGMFTRDQPPGYKLNSYGFHPYYMALENEGNAHGVLLLNSNGMDVTFQPTPALTYRTIGGILDFYMFLGPTPEIATRQYHEVIGFPVMPPYWALGFQLCRYGYRNTSEIEQLYNDMVAANIPYDVQYTDINYMERQLDFTIGERFKTLPEFVDRIRKDGMKYIVILAPAISGNETQPYPAFERGIQKDVFVKWPNTNDICWPKVWPDLPNVTIDETITEDEAVNASRAHVAFPDFFRNSTLEWWAREIYDFYNEKMKFDGLWIDMNEPSSFGIQMGGKVLNECRRMMTLNYPPVFSPELRVKEGEGASISEAMCMETEHILIDGSSVLQYDVHNLYGWSQVKPTLDALQNTTGLRGIVISRSTYPTTGRWGGHWLGDNYTTWDNLEKSLIGMLELNLFGIPYIGADICGVFHDSGYPSLYFVGIQVGAFYPYPRESPTINFTRSQDPVSWMKLLLQMSKKVLEIRYTLLPYFYTQMHEAHAHGGTVIRPLMHEFFDDKETWEIYKQFLWGPAFMVTPVVEPFRTSVTGYVPKARWFDYHTGADIKLKGILHTFSAPFDTINLHVRGGYILPCQEPARNTHLSRQNYMKLIVAADDNQMAQGTLFGDDGESIDTYERGQYTSIQFNLNQTTLTSTVLANGYKNKQEMRLGSIHIWGKGTLRISNANLVYGGRKHQPPFTQEEAKETLIFDLKNMNVTLDEPIQITWS
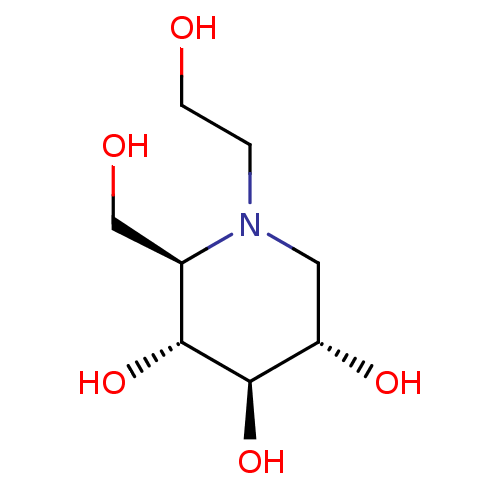
Inhibitor
Name:
BDBM50242271
Synonyms:
(2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)piperidine-3,4,5-triol | (2R,3R,4R,5S)-1-ethoxy-2-(hydroxymethyl)piperidine-3,4,5-triol | BAY-M-1099 | CHEMBL1561 | Glyset | MIGLITOL | N-Hydroxyethyl-1-deoxynojirimycin | cid_441314
Type:
Small organic molecule
Emp. Form.:
C8H17NO5
Mol. Mass.:
207.2243
SMILES:
OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r|