Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Lysine-specific histone demethylase 1A
Ligand
BDBM50158882
Substrate
n/a
Meas. Tech.
ChEMBL_1566028 (CHEMBL3791186)
Ki
210±n/a nM
Citation
 McAllister, TE; England, KS; Hopkinson, RJ; Brennan, PE; Kawamura, A; Schofield, CJ Recent Progress in Histone Demethylase Inhibitors. J Med Chem 59:1308-29 (2016) [PubMed] Article
McAllister, TE; England, KS; Hopkinson, RJ; Brennan, PE; Kawamura, A; Schofield, CJ Recent Progress in Histone Demethylase Inhibitors. J Med Chem 59:1308-29 (2016) [PubMed] Article More Info.:
Target
Name:
Lysine-specific histone demethylase 1A
Synonyms:
AOF2 | BRAF35-HDAC complex protein BHC110 | Flavin-containing amine oxidase domain-containing protein 2 | KDM1 | KDM1A | KDM1A_HUMAN | KIAA0601 | LSD1 | Lysine-specific demethylase 1 (LSD1) | Lysine-specific histone demethylase 1 | Lysine-specific histone demethylase 1 (LSD1)
Type:
Enzyme
Mol. Mass.:
92901.01
Organism:
Homo sapiens (Human)
Description:
O60341
Residue:
852
Sequence:
MLSGKKAAAAAAAAAAAATGTEAGPGTAGGSENGSEVAAQPAGLSGPAEVGPGAVGERTPRKKEPPRASPPGGLAEPPGSAGPQAGPTVVPGSATPMETGIAETPEGRRTSRRKRAKVEYREMDESLANLSEDEYYSEEERNAKAEKEKKLPPPPPQAPPEEENESEPEEPSGVEGAAFQSRLPHDRMTSQEAACFPDIISGPQQTQKVFLFIRNRTLQLWLDNPKIQLTFEATLQQLEAPYNSDTVLVHRVHSYLERHGLINFGIYKRIKPLPTKKTGKVIIIGSGVSGLAAARQLQSFGMDVTLLEARDRVGGRVATFRKGNYVADLGAMVVTGLGGNPMAVVSKQVNMELAKIKQKCPLYEANGQAVPKEKDEMVEQEFNRLLEATSYLSHQLDFNVLNNKPVSLGQALEVVIQLQEKHVKDEQIEHWKKIVKTQEELKELLNKMVNLKEKIKELHQQYKEASEVKPPRDITAEFLVKSKHRDLTALCKEYDELAETQGKLEEKLQELEANPPSDVYLSSRDRQILDWHFANLEFANATPLSTLSLKHWDQDDDFEFTGSHLTVRNGYSCVPVALAEGLDIKLNTAVRQVRYTASGCEVIAVNTRSTSQTFIYKCDAVLCTLPLGVLKQQPPAVQFVPPLPEWKTSAVQRMGFGNLNKVVLCFDRVFWDPSVNLFGHVGSTTASRGELFLFWNLYKAPILLALVAGEAAGIMENISDDVIVGRCLAILKGIFGSSAVPQPKETVVSRWRADPWARGSYSYVAAGSSGNDYDLMAQPITPGPSIPGAPQPIPRLFFAGEHTIRNYPATVHGALLSGLREAGRIADQFLGAMYTLPRQATPGVPAQQSPSM
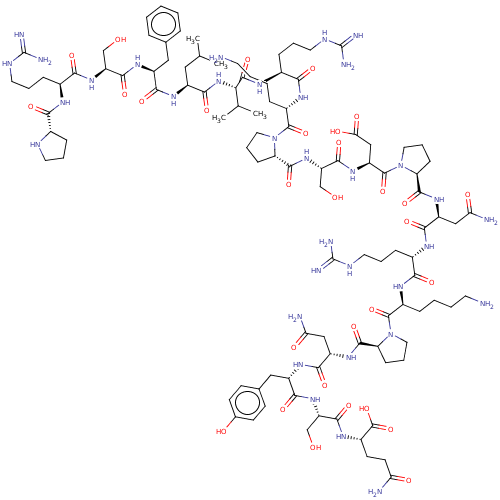
Inhibitor
Name:
BDBM50158882
Synonyms:
CHEMBL3786209
Type:
Small organic molecule
Emp. Form.:
C105H168N34O30
Mol. Mass.:
2386.6672
SMILES:
CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r|