Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Type-1 angiotensin II receptor A/B
Ligand
BDBM50230881
Substrate
n/a
Meas. Tech.
ChEMBL_34973 (CHEMBL649502)
IC50
3.3±n/a nM
Citation
 Sircar, I; Hodges, JC; Quin, J; Bunker, AM; Winters, RT; Edmunds, JJ; Kostlan, CR; Connolly, C; Kesten, SJ; Hamby, JM Nonpeptide angiotensin II receptor antagonists. 2. Design, synthesis, and structure-activity relationships of 2-alkyl-4-(1H-pyrrol-1-yl)-1H-imidazole derivatives: profile of 2-propyl-1-[[2'-(1H-tetrazol-5-yl)-[1,1' -biphenyl]-4-yl]-methyl]-4-[2-(trifluoroacetyl)-1H-pyrrol-1-yl]-1H- imidazole-5-car J Med Chem 36:2253-65 (1993) [PubMed] Article
Sircar, I; Hodges, JC; Quin, J; Bunker, AM; Winters, RT; Edmunds, JJ; Kostlan, CR; Connolly, C; Kesten, SJ; Hamby, JM Nonpeptide angiotensin II receptor antagonists. 2. Design, synthesis, and structure-activity relationships of 2-alkyl-4-(1H-pyrrol-1-yl)-1H-imidazole derivatives: profile of 2-propyl-1-[[2'-(1H-tetrazol-5-yl)-[1,1' -biphenyl]-4-yl]-methyl]-4-[2-(trifluoroacetyl)-1H-pyrrol-1-yl]-1H- imidazole-5-car J Med Chem 36:2253-65 (1993) [PubMed] Article More Info.:
Target
Name:
Type-1 angiotensin II receptor A/B
Synonyms:
Angiotensin II receptor (AT-1) type-1 | Type-1A/Type-1B angiotensin II receptor
Type:
n/a
Mol. Mass.:
n/a
Description:
ASSAY_ID of ChEMBL is 901964
Components:
This complex has 2 components.
Component 1
Name:
Type-1 angiotensin II receptor B
Synonyms:
AGTRB_RAT | AT3 | Agtr1 | Agtr1b | Angiotensin II AT1B | Angiotensin II receptor (AT-1) type-1 | Angiotensin II type 1b (AT-1b) receptor | At1b | Type-1B angiotensin II receptor
Type:
Enzyme Catalytic Domain
Mol. Mass.:
40929.44
Organism:
RAT
Description:
Angiotensin II AT1B 0 RAT::P29089
Residue:
359
Sequence:
MTLNSSTEDGIKRIQDDCPKAGRHNYIFVMIPTLYSIIFVVGIFGNSLVVIVIYFYMKLKTVASVFLLNLALADLCFLLTLPLWAVYTAMEYRWPFGNHLCKIASASVSFNLYASVFLLTCLSIDRYLAIVHPMKSRLRRTMLVAKVTCIIIWLMAGLASLPAVIYRNVYFIENTNITVCAFHYESQNSTLPIGLGLTKNILGFVFPFLIILTSYTLIWKALKKAYKIQKNTPRNDDIFRIIMAIVLFFFFSWVPHQIFTFLDVLIQLGIIRDCEIADIVDTAMPITICIAYFNNCLNPLFYGFLGKKFKKYFLQLLKYIPPTAKSHAGLSTKMSTLSYRPSDNMSSSAKKSASFFEVE
Component 2
Name:
Type-1 angiotensin II receptor A
Synonyms:
AGTRA_RAT | ANGIOTENSIN AT1 | Agtr1 | Agtr1a | Angiotensin II AT1 | Angiotensin II AT1A | Angiotensin II receptor (AT-1) type-1 | At1a | Type-1A angiotensin II receptor
Type:
Enzyme Catalytic Domain
Mol. Mass.:
40910.53
Organism:
RAT
Description:
ANGIOTENSIN AT1 AGTR1 RAT::P25095
Residue:
359
Sequence:
MALNSSAEDGIKRIQDDCPKAGRHSYIFVMIPTLYSIIFVVGIFGNSLVVIVIYFYMKLKTVASVFLLNLALADLCFLLTLPLWAVYTAMEYRWPFGNHLCKIASASVSFNLYASVFLLTCLSIDRYLAIVHPMKSRLRRTMLVAKVTCIIIWLMAGLASLPAVIHRNVYFIENTNITVCAFHYESRNSTLPIGLGLTKNILGFLFPFLIILTSYTLIWKALKKAYEIQKNKPRNDDIFRIIMAIVLFFFFSWVPHQIFTFLDVLIQLGVIHDCKISDIVDTAMPITICIAYFNNCLNPLFYGFLGKKFKKYFLQLLKYIPPKAKSHSSLSTKMSTLSYRPSDNMSSSAKKPASCFEVE
Inhibitor
Name:
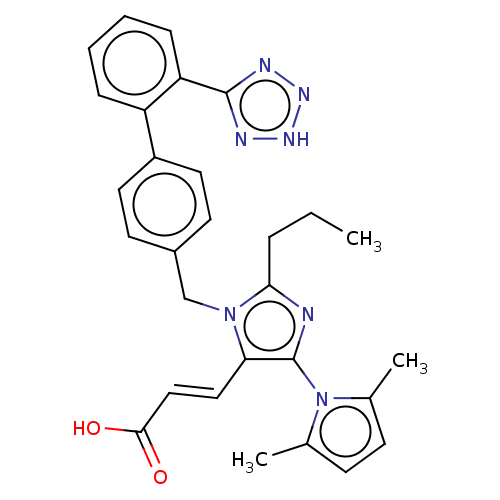
BDBM50230881
Synonyms:
CHEMBL308779
Type:
Small organic molecule
Emp. Form.:
C29H29N7O2
Mol. Mass.:
507.5863
SMILES:
CCCc1nc(c(\C=C\C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1c(C)ccc1C |(11.3,-11.62,;12.77,-12.09,;13.92,-11.05,;15.38,-11.53,;15.86,-12.98,;17.39,-12.98,;17.87,-11.49,;19.34,-11.05,;19.65,-9.55,;21.09,-9.07,;22.24,-10.09,;21.41,-7.57,;16.62,-10.62,;16.62,-9.07,;15.28,-8.3,;15.26,-6.74,;13.92,-6,;12.6,-6.77,;12.6,-8.3,;13.94,-9.07,;11.26,-6.01,;9.93,-6.77,;8.59,-6.01,;8.59,-4.44,;9.93,-3.68,;11.26,-4.44,;12.58,-3.67,;12.74,-2.14,;14.24,-1.8,;15.02,-3.14,;13.98,-4.28,;18.32,-14.23,;19.85,-14.2,;20.74,-12.96,;20.33,-15.67,;19.08,-16.6,;17.84,-15.69,;16.39,-16.17,)|