Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Neuronal acetylcholine receptor subunit alpha-4
Ligand
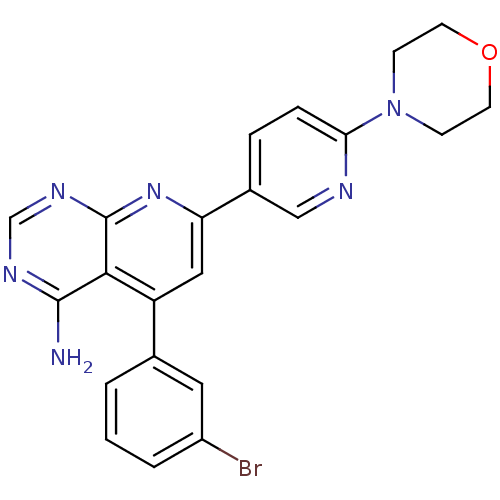
BDBM50094703
Substrate
n/a
Ki
>10000±n/a nM
Comments
PDSP_2986
Citation
 Jarvis, MF; Yu, H; Kohlhaas, K; Alexander, K; Lee, CH; Jiang, M; Bhagwat, SS; Williams, M; Kowaluk, EA ABT-702 (4-amino-5-(3-bromophenyl)-7-(6-morpholinopyridin-3-yl)pyrido[2, 3-d]pyrimidine), a novel orally effective adenosine kinase inhibitor with analgesic and anti-inflammatory properties: I. In vitro characterization and acute antinociceptive effects in the mouse. J Pharmacol Exp Ther 295:1156-64 (2000) [PubMed]
Jarvis, MF; Yu, H; Kohlhaas, K; Alexander, K; Lee, CH; Jiang, M; Bhagwat, SS; Williams, M; Kowaluk, EA ABT-702 (4-amino-5-(3-bromophenyl)-7-(6-morpholinopyridin-3-yl)pyrido[2, 3-d]pyrimidine), a novel orally effective adenosine kinase inhibitor with analgesic and anti-inflammatory properties: I. In vitro characterization and acute antinociceptive effects in the mouse. J Pharmacol Exp Ther 295:1156-64 (2000) [PubMed] More Info.:
Target
Name:
Neuronal acetylcholine receptor subunit alpha-4
Synonyms:
ACHA4_RAT | Acra4 | Cholinergic, Nicotinic Alpha4Beta2 | Cholinergic, Nicotinic Alpha4Beta4 | Chrna4 | Neuronal acetylcholine receptor | Neuronal acetylcholine receptor (alpha4beta2 nAChR) | Neuronal acetylcholine receptor protein alpha-4 subunit | Neuronal acetylcholine receptor subunit alpha 4 beta 2 | Neuronal acetylcholine receptor subunit alpha-4
Type:
Enzyme
Mol. Mass.:
70196.44
Organism:
Rattus norvegicus (Rat)
Description:
P09483
Residue:
630
Sequence:
MANSGTGAPPPLLLLPLLLLLGTGLLPASSHIETRAHAEERLLKRLFSGYNKWSRPVANISDVVLVRFGLSIAQLIDVDEKNQMMTTNVWVKQEWHDYKLRWDPGDYENVTSIRIPSELIWRPDIVLYNNADGDFAVTHLTKAHLFYDGRVQWTPPAIYKSSCSIDVTFFPFDQQNCTMKFGSWTYDKAKIDLVSMHSRVDQLDFWESGEWVIVDAVGTYNTRKYECCAEIYPDITYAFIIRRLPLFYTINLIIPCLLISCLTVLVFYLPSECGEKVTLCISVLLSLTVFLLLITEIIPSPTSLVIPLIGEYLLFTMIFVTLSIVITVFVLNVHHRSPRTHTMPAWVRRVFLDIVPRLLFMKRPSVVKDNCRRLIESMHKMANAPRFWPEPVGEPGILSDICNQGLSPAPTFCNPTDTAVETQPTCRSPPLEVPDLKTSEVEKASPCPSPGSCPPPKSSSGAPMLIKARSLSVQHVPSSQEAAEDGIRCRSRSIQYCVSQDGAASLADSKPTSSPTSLKARPSQLPVSDQASPCKCTCKEPSPVSPVTVLKAGGTKAPPQHLPLSPALTRAVEGVQYIADHLKAEDTDFSVKEDWKYVAMVIDRIFLWMFIIVCLLGTVGLFLPPWLAAC
Inhibitor
Name:
BDBM50094703
Synonyms:
5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-yl)-pyrido[2,3-d]pyrimidin-4-ylamine | 5-(3-bromophenyl)-7-(6-morpholin-4-ylpyridin-3-yl)pyrido[2,3-d]pyrimidin-4-ylamine | ABT-702 | CHEMBL66089
Type:
Small organic molecule
Emp. Form.:
C22H19BrN6O
Mol. Mass.:
463.33
SMILES:
Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1