Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Leucine-rich repeat serine/threonine-protein kinase 2 [970-2527]
Ligand
BDBM50322535
Substrate
n/a
Meas. Tech.
LRRK2 Kinase Assay
pH
7.4±n/a
Temperature
298.15±n/a K
IC50
1.6e+2± 1e+1 nM
Comments
extracted
Citation
More Info.:
Target
Name:
Leucine-rich repeat serine/threonine-protein kinase 2 [970-2527]
Synonyms:
LRRK2 | LRRK2_HUMAN | Leucine-rich repeat kinase 2 (LRRK2 t-WT) | Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) | PARK8
Type:
Protein
Mol. Mass.:
177317.56
Organism:
Homo sapiens (Human)
Description:
Human LRRK2 variant (970-2527aa)
Residue:
1558
Sequence:
HSDSISSLASEREYITSLDLSANELRDIDALSQKCCISVHLEHLEKLELHQNALTSFPQQLCETLKSLTHLDLHSNKFTSFPSYLLKMSCIANLDVSRNDIGPSVVLDPTVKCPTLKQFNLSYNQLSFVPENLTDVVEKLEQLILEGNKISGICSPLRLKELKILNLSKNHISSLSENFLEACPKVESFSARMNFLAAMPFLPPSMTILKLSQNKFSCIPEAILNLPHLRSLDMSSNDIQYLPGPAHWKSLNLRELLFSHNQISILDLSEKAYLWSRVEKLHLSHNKLKEIPPEIGCLENLTSLDVSYNLELRSFPNEMGKLSKIWDLPLDELHLNFDFKHIGCKAKDIIRFLQQRLKKAVPYNRMKLMIVGNTGSGKTTLLQQLMKTKKSDLGMQSATVGIDVKDWPIQIRDKRKRDLVLNVWDFAGREEFYSTHPHFMTQRALYLAVYDLSKGQAEVDAMKPWLFNIKARASSSPVILVGTHLDVSDEKQRKACMSKITKELLNKRGFPAIRDYHFVNATEESDALAKLRKTIINESLNFKIRDQLVVGQLIPDCYVELEKIILSERKNVPIEFPVIDRKRLLQLVRENQLQLDENELPHAVHFLNESGVLLHFQDPALQLSDLYFVEPKWLCKIMAQILTVKVEGCPKHPKGIISRRDVEKFLSKKRKFPKNYMSQYFKLLEKFQIALPIGEEYLLVPSSLSDHRPVIELPHCENSEIIIRLYEMPYFPMGFWSRLINRLLEISPYMLSGRERALRPNRMYWRQGIYLNWSPEAYCLVGSEVLDNHPESFLKITVPSCRKGCILLGQVVDHIDSLMEEWFPGLLEIDICGEGETLLKKWALYSFNDGEEHQKILLDDLMKKAEEGDLLVNPDQPRLTIPISQIAPDLILADLPRNIMLNNDELEFEQAPEFLLGDGSFGSVYRAAYEGEEVAVKIFNKHTSLRLLRQELVVLCHLHHPSLISLLAAGIRPRMLVMELASKGSLDRLLQQDKASLTRTLQHRIALHVADGLRYLHSAMIIYRDLKPHNVLLFTLYPNAAIIAKIADYGIAQYCCRMGIKTSEGTPGFRAPEVARGNVIYNQQADVYSFGLLLYDILTTGGRIVEGLKFPNEFDELEIQGKLPDPVKEYGCAPWPMVEKLIKQCLKENPQERPTSAQVFDILNSAELVCLTRRILLPKNVIVECMVATHHNSRNASIWLGCGHTDRGQLSFLDLNTEGYTSEEVADSRILCLALVHLPVEKESWIVSGTQSGTLLVINTEDGKKRHTLEKMTDSVTCLYCNSFSKQSKQKNFLLVGTADGKLAIFEDKTVKLKGAAPLKILNIGNVSTPLMCLSESTNSTERNVMWGGCGTKIFSFSNDFTIQKLIETRTSQLFSYAAFSDSNIITVVVDTALYIAKQNSPVVEVWDKKTEKLCGLIDCVHFLREVMVKENKESKHKMSYSGRVKTLCLQKNTALWIGTGGGHILLLDLSTRRLIRVIYNFCNSVRVMMTAQLGSLKNVMLVLGYNRKNTEGTQKQKEIQSCLTVWDINLPHEVQNLEKHIEVRKELAEKMRRTSVE
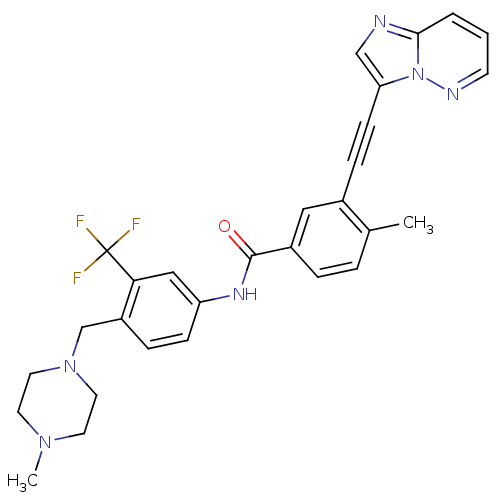
Inhibitor
Name:
BDBM50322535
Synonyms:
3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide | 3-[2-(Imidazo[1,2-b]pyridazin-3-yl)ethynyl]-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide | CHEMBL1171837 | PONATINIB | US10464902, Ponatinib | US9255107, AP24534
Type:
Small organic molecule
Emp. Form.:
C29H27F3N6O
Mol. Mass.:
532.5595
SMILES:
CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1