Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Fibroblast growth factor receptor 2
Ligand
BDBM443226
Substrate
n/a
Meas. Tech.
Determination of the Effect of the Compounds of the Present Invention Against FGFR Kinase Activity
IC50
405±n/a nM
Citation
More Info.:
Target
Name:
Fibroblast growth factor receptor 2
Synonyms:
BEK | CD_antigen=CD332 | FGFR-2 | FGFR-2 Tyrosine Kinase | FGFR2 | FGFR2_HUMAN | Fibroblast growth factor receptor 2 (FGFR2) | Fibroblast growth factor receptor 2 precursor | KGFR | KSAM | Keratinocyte growth factor receptor | Keratinocyte growth factor receptor 2 | VEGF-receptor 2 and Fibroblast growth factor receptor 2
Type:
Enzyme
Mol. Mass.:
92015.45
Organism:
Homo sapiens (Human)
Description:
P21802
Residue:
821
Sequence:
MVSWGRFICLVVVTMATLSLARPSFSLVEDTTLEPEEPPTKYQISQPEVYVAAPGESLEVRCLLKDAAVISWTKDGVHLGPNNRTVLIGEYLQIKGATPRDSGLYACTASRTVDSETWYFMVNVTDAISSGDDEDDTDGAEDFVSENSNNKRAPYWTNTEKMEKRLHAVPAANTVKFRCPAGGNPMPTMRWLKNGKEFKQEHRIGGYKVRNQHWSLIMESVVPSDKGNYTCVVENEYGSINHTYHLDVVERSPHRPILQAGLPANASTVVGGDVEFVCKVYSDAQPHIQWIKHVEKNGSKYGPDGLPYLKVLKAAGVNTTDKEIEVLYIRNVTFEDAGEYTCLAGNSIGISFHSAWLTVLPAPGREKEITASPDYLEIAIYCIGVFLIACMVVTVILCRMKNTTKKPDFSSQPAVHKLTKRIPLRRQVTVSAESSSSMNSNTPLVRITTRLSSTADTPMLAGVSEYELPEDPKWEFPRDKLTLGKPLGEGCFGQVVMAEAVGIDKDKPKEAVTVAVKMLKDDATEKDLSDLVSEMEMMKMIGKHKNIINLLGACTQDGPLYVIVEYASKGNLREYLRARRPPGMEYSYDINRVPEEQMTFKDLVSCTYQLARGMEYLASQKCIHRDLAARNVLVTENNVMKIADFGLARDINNIDYYKKTTNGRLPVKWMAPEALFDRVYTHQSDVWSFGVLMWEIFTLGGSPYPGIPVEELFKLLKEGHRMDKPANCTNELYMMMRDCWHAVPSQRPTFKQLVEDLDRILTLTTNEEYLDLSQPLEQYSPSYPDTRSSCSSGDDSVFSPDPMPYEPCLPQYPHINGSVKT
Inhibitor
Name:
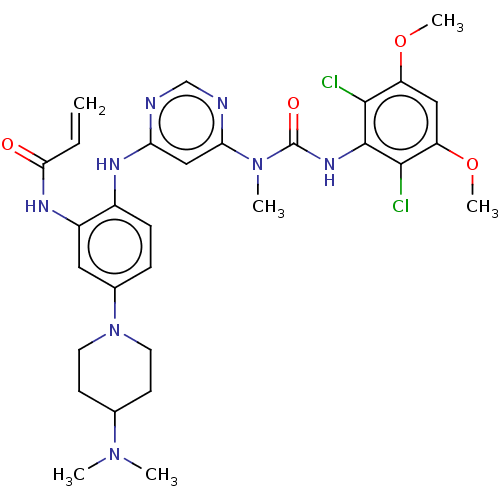
BDBM443226
Synonyms:
N-(2-((6-(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-methylureido)pyrimidin-4-yl)amino)-5-(4-(dimethylamino)piperidin-1-yl)phenyl)acrylamide | US10654836, Example 7 | US11001572, Example 7
Type:
Small organic molecule
Emp. Form.:
C30H36Cl2N8O4
Mol. Mass.:
643.564
SMILES:
COc1cc(OC)c(Cl)c(NC(=O)N(C)c2cc(Nc3ccc(cc3NC(=O)C=C)N3CCC(CC3)N(C)C)ncn2)c1Cl