Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Interleukin-1 receptor-associated kinase 4
Ligand
BDBM504428
Substrate
n/a
Meas. Tech.
Biochemical Assay
IC50
65±n/a nM
Citation
 Crew, AP; Araujo, E Compounds and methods for the targeted degradation of interleukin-1 receptor- associated kinase 4 polypeptides US Patent US11065231 Publication Date 7/20/2021
Crew, AP; Araujo, E Compounds and methods for the targeted degradation of interleukin-1 receptor- associated kinase 4 polypeptides US Patent US11065231 Publication Date 7/20/2021 More Info.:
Target
Name:
Interleukin-1 receptor-associated kinase 4
Synonyms:
IRAK-4 | IRAK4 | IRAK4_HUMAN | Interleukin-1 receptor-associated kinase 4 (IRAK-4) | Interleukin-1 receptor-associated kinase 4 (IRAK4) | Renal carcinoma antigen NY-REN-64
Type:
Protein
Mol. Mass.:
51519.08
Organism:
Homo sapiens (Human)
Description:
Q9NWZ3
Residue:
460
Sequence:
MNKPITPSTYVRCLNVGLIRKLSDFIDPQEGWKKLAVAIKKPSGDDRYNQFHIRRFEALLQTGKSPTSELLFDWGTTNCTVGDLVDLLIQNEFFAPASLLLPDAVPKTANTLPSKEAITVQQKQMPFCDKDRTLMTPVQNLEQSYMPPDSSSPENKSLEVSDTRFHSFSFYELKNVTNNFDERPISVGGNKMGEGGFGVVYKGYVNNTTVAVKKLAAMVDITTEELKQQFDQEIKVMAKCQHENLVELLGFSSDGDDLCLVYVYMPNGSLLDRLSCLDGTPPLSWHMRCKIAQGAANGINFLHENHHIHRDIKSANILLDEAFTAKISDFGLARASEKFAQTVMTSRIVGTTAYMAPEALRGEITPKSDIYSFGVVLLEIITGLPAVDEHREPQLLLDIKEEIEDEEKTIEDYIDKKMNDADSTSVEAMYSVASQCLHEKKNKRPDIKKVQQLLQEMTAS
Inhibitor
Name:
BDBM504428
Synonyms:
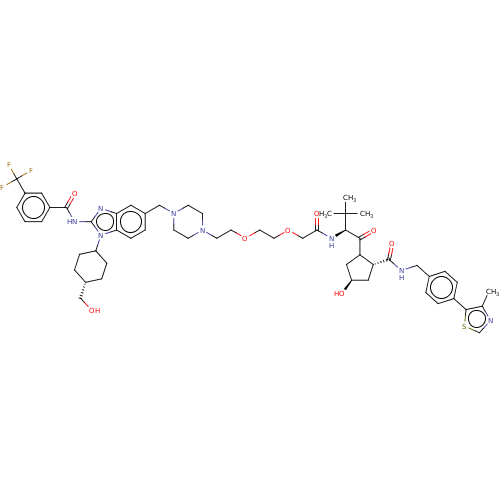
(2S,4R)-4-hydroxy-1-((S)-2- (2-(2-(2-(4-((1-((1SR,4SR)- 4- (hydroxymethyl)cyclohexyl)- 2-(3- (trifluoromethyl)benzamido)- 1H-benzo[d]imidazol-5- yl)methyl)piperazin-1- yl)ethoxy)ethoxy)acetamido)- 3,3-dimethylbutanoyl)-N- (4-(4-methylthiazol-5- yl)benzyl)pyrrolidine-2- carboxamide | US11065231, Example 12
Type:
Small organic molecule
Emp. Form.:
C56H71F3N8O8S
Mol. Mass.:
1073.272
SMILES:
Cc1ncsc1-c1ccc(CNC(=O)[C@@H]2C[C@@H](O)CC2C(=O)[C@@H](NC(=O)COCCOCCN2CCN(Cc3ccc4n(C5CC[C@H](CO)CC5)c(NC(=O)c5cccc(c5)C(F)(F)F)nc4c3)CC2)C(C)(C)C)cc1 |r,wU:46.48,22.24,14.14,wD:16.17,(14.21,-8.49,;12.97,-7.58,;11.5,-8.06,;10.6,-6.82,;11.5,-5.57,;12.96,-6.04,;13.99,-4.9,;13.52,-3.44,;14.55,-2.29,;16.06,-2.61,;17.09,-1.47,;16.61,-0,;17.64,1.14,;19.15,.82,;17.16,2.61,;18.07,3.85,;17.16,5.1,;17.64,6.56,;15.7,4.62,;15.7,3.08,;14.37,2.31,;14.37,.77,;13.03,3.08,;11.7,2.31,;10.36,3.08,;10.36,4.62,;9.03,2.31,;7.7,3.08,;6.36,2.31,;5.03,3.08,;3.7,2.31,;2.36,3.08,;1.03,2.31,;-.3,3.08,;-1.64,2.31,;-2.97,3.08,;-2.97,4.62,;-4.31,5.39,;-5.64,4.62,;-5.64,3.08,;-6.97,2.31,;-8.31,3.08,;-9.77,2.61,;-10.25,1.14,;-11.75,.82,;-12.23,-.64,;-11.2,-1.79,;-11.67,-3.25,;-13.18,-3.57,;-9.69,-1.47,;-9.22,-0,;-10.68,3.85,;-12.22,3.85,;-12.99,5.19,;-12.22,6.52,;-14.53,5.19,;-15.3,3.85,;-16.84,3.85,;-17.61,5.19,;-16.84,6.52,;-15.3,6.52,;-17.61,7.85,;-19.15,7.85,;-18.38,9.19,;-16.84,9.19,;-9.77,5.1,;-8.31,4.62,;-6.97,5.39,;-1.64,5.39,;-.3,4.62,;13.03,4.62,;11.7,5.39,;14.37,5.39,;13.03,6.16,;16.53,-4.08,;15.5,-5.22,)|