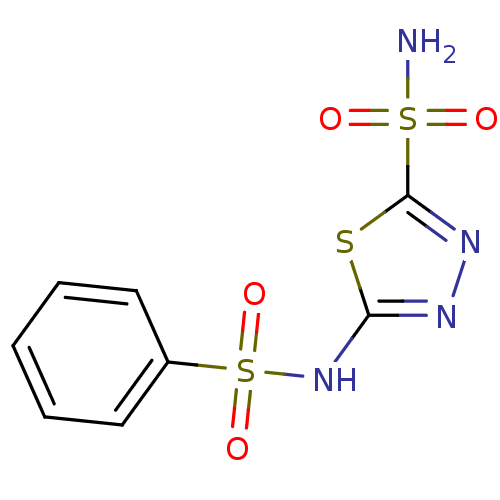
BDBM10886 2-N-benzene-1,3,4-thiadiazole-2,5-disulfonamide::BZA1::Benzolamide::Benzolamide (BZA)::CHEMBL73962
SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccccc2)s1
InChI Key InChIKey=PWDGTQXZLNDOKS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 10886
Found 4 hits for monomerid = 10886
Affinity DataKi: 9nMAssay Description:Inhibition of human recombinant cytosolic isozyme CA II by stopped-flow CO2 hydrase methodMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibition of human recombinant cytosolic isozyme CA I by stopped-flow CO2 hydrase methodMore data for this Ligand-Target Pair
Affinity DataKi: 47nMAssay Description:Inhibition of catalytic domain of human recombinant CA IXMore data for this Ligand-Target Pair
Affinity DataKi: 93nMAssay Description:Inhibition of full length human recombinant CA VIMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)