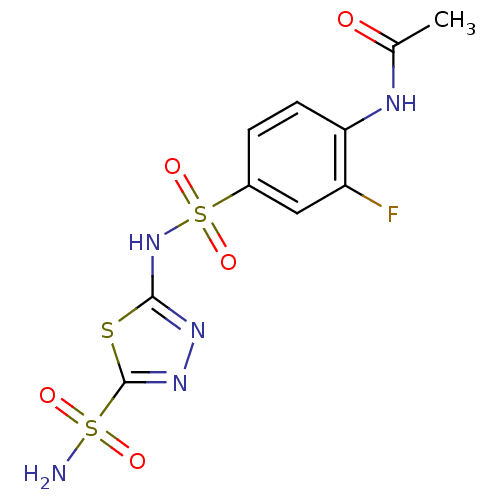
BDBM11616 5-(4-Acetamido-3-fluorobenzenesulfonamido)-1,3,4-thiadiazole-2-sulfonamide::N-{2-fluoro-4-[(5-sulfamoyl-1,3,4-thiadiazol-2-yl)sulfamoyl]phenyl}acetamide::aminobenzolamide 16b::aminobenzolamide deriv. 40
SMILES CC(=O)Nc1ccc(cc1F)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O
InChI Key InChIKey=ATYLCJOUAUSNKE-UHFFFAOYSA-N
Data 8 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 11616
Found 8 hits for monomerid = 11616
Affinity DataKi: 1nMAssay Description:Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 4(Bos taurus (bovine))
University Of Agricultural Sciences And Veterinary Medicine
University Of Agricultural Sciences And Veterinary Medicine
Affinity DataKi: 4nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 4(Bos taurus (bovine))
University Of Agricultural Sciences And Veterinary Medicine
University Of Agricultural Sciences And Veterinary Medicine
Affinity DataKi: 4nMAssay Description:Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 1(Homo sapiens (Human))
University Of Agricultural Sciences And Veterinary Medicine
University Of Agricultural Sciences And Veterinary Medicine
Affinity DataKi: 5.70nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 1(Homo sapiens (Human))
University Of Agricultural Sciences And Veterinary Medicine
University Of Agricultural Sciences And Veterinary Medicine
Affinity DataKi: 6nMAssay Description:Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 9(Homo sapiens (Human))
University Of Agricultural Sciences And Veterinary Medicine
University Of Agricultural Sciences And Veterinary Medicine
Affinity DataKi: 79nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair