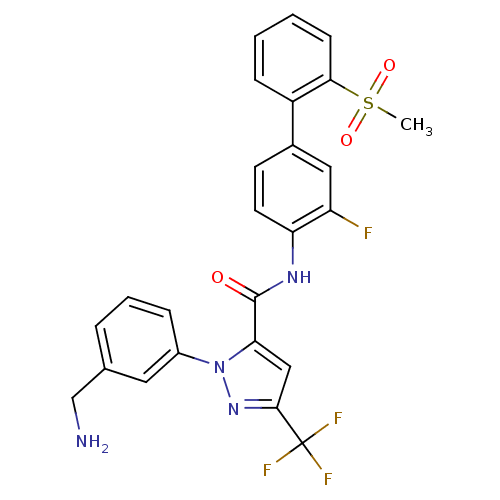
BDBM12657 1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsulfonyl)-[1,1-biphenyl]-4-yl]-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide::1-[3-(aminomethyl)phenyl]-N-[2-fluoro-4-(2-methanesulfonylphenyl)phenyl]-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide::CHEMBL559015::DPC 423::DPC423
SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2cccc(CN)c2)C(F)(F)F)c(F)c1
InChI Key InChIKey=ZLUOAFAJSUPHOG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 39 hits for monomerid = 12657
Found 39 hits for monomerid = 12657
Affinity DataKi: 0.150nMAssay Description:Tested in vitro for inhibition of human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 0.150nM ΔG°: -13.3kcal/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Tested in vitro for inhibition of rabbit Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:In vitro activity against rabbit factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nM ΔG°: -12.9kcal/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Tested in vitro for inhibition of human trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:In vitro activity against human trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 61nMAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 61nMAssay Description:In vitro activity against human Plasma kallikrein.More data for this Ligand-Target Pair
Affinity DataKi: 61nMAssay Description:Tested in vitro for inhibition of human plasma KallikrieneMore data for this Ligand-Target Pair
Affinity DataKi: 150nMAssay Description:Binding affinity against human coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 1.80E+3nMAssay Description:In vitro activity against human Activated protein CMore data for this Ligand-Target Pair
Affinity DataKi: 1.80E+3nMAssay Description:Tested in vitro for inhibition of human activated protein CMore data for this Ligand-Target Pair
Affinity DataKi: 2.20E+3nMAssay Description:Tested in vitro for inhibition of human Coagulation factor IXMore data for this Ligand-Target Pair
Affinity DataKi: 2.20E+3nMAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 2.20E+3nMAssay Description:In vitro activity against human factor IXaMore data for this Ligand-Target Pair
Affinity DataKi: 6.00E+3nMAssay Description:In vitro activity against human thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 6.00E+3nMAssay Description:Inhibition of human factor 2aMore data for this Ligand-Target Pair
Affinity DataKi: 6.00E+3nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 6.00E+3nMAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 6.00E+3nMAssay Description:In vitro for inhibition of human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+4nMAssay Description:The compound was tested for inhibition of human coagulation factor VIIMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+4nMAssay Description:Tested in vitro for inhibition of human Coagulation factor VIIMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+4nMAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: >1.70E+4nMAssay Description:In vitro activity against human Chymotrypsinogen B1More data for this Ligand-Target Pair
Affinity DataKi: >1.70E+4nMAssay Description:Tested in vitro for inhibition of human ChymotrypsinogenMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: >1.90E+4nMAssay Description:Tested in vitro for inhibition of human Urokinase-type plasminogen activatorMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: >1.90E+4nMAssay Description:In vitro activity against human urokinaseMore data for this Ligand-Target Pair
Affinity DataKi: >3.50E+4nMAssay Description:Tested in vitro for inhibition of human plasminMore data for this Ligand-Target Pair
Affinity DataKi: >3.50E+4nMAssay Description:In vitro activity against human plasminMore data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Dupont Pharmaceuticals
Curated by ChEMBL
Dupont Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >4.50E+4nMAssay Description:In vitro activity against human tissue plasminogen activatorMore data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Dupont Pharmaceuticals
Curated by ChEMBL
Dupont Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >4.50E+4nMAssay Description:Tested in vitro for inhibition of human Tissue type plasminogen activatorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human Trypsin using S-2238 chromogenic substrate assessed as hydrolysis by microplate readerMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human Thrombin using S-2222 chromogenic substrate assessed as hydrolysis by microplate readerMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Inhibition of human factor 10a using S-2222 chromogenic substrate assessed as hydrolysis by microplate readerMore data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+4nMAssay Description:In vitro activity against human Complement factor IMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)