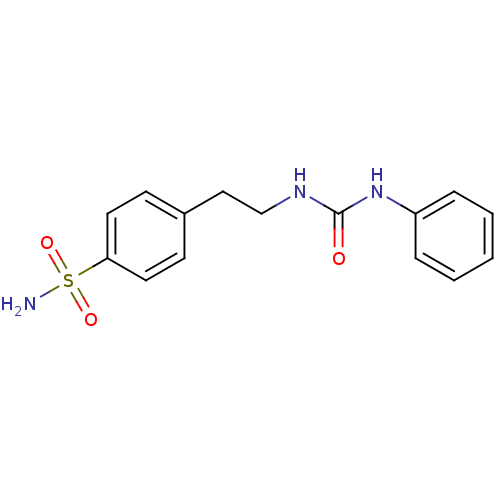
BDBM16656 1-phenyl-3-[2-(4-sulfamoylphenyl)ethyl]urea::CHEMBL22374::aromatic sulfonamide compound 21
SMILES NS(=O)(=O)c1ccc(CCNC(=O)Nc2ccccc2)cc1
InChI Key InChIKey=CQMWNAHSFVPGJL-UHFFFAOYSA-N
Data 8 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 16656
Found 8 hits for monomerid = 16656
Affinity DataKi: 13nMAssay Description:Inhibitiory activity against human Carbonic anhydrase XII (hCA XII)More data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibitiory activity against human Carbonic anhydrase IX (hCA IX)More data for this Ligand-Target Pair
Affinity DataKi: 75nMAssay Description:Inhibitiory activity against human Carbonic anhydrase II (hCA II)More data for this Ligand-Target Pair
Affinity DataKi: 75nMAssay Description:Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt...More data for this Ligand-Target Pair
Affinity DataKi: 150nMAssay Description:Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt...More data for this Ligand-Target Pair
Affinity DataKi: 430nMAssay Description:Inhibitiory activity against human Carbonic anhydrase I (hCA I)More data for this Ligand-Target Pair
Affinity DataKi: 430nMAssay Description:Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt...More data for this Ligand-Target Pair