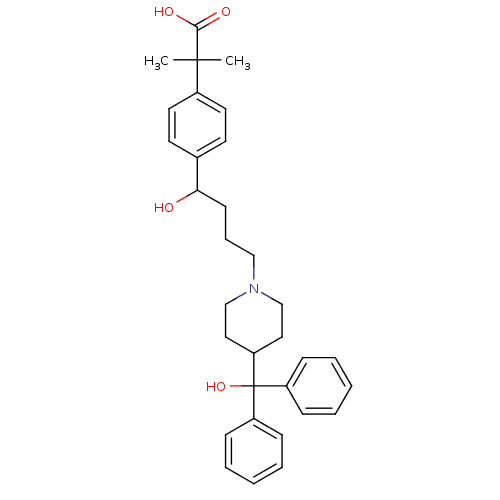
BDBM22874 2-(4-{1-hydroxy-4-[4-(hydroxydiphenylmethyl)piperidin-1-yl]butyl}phenyl)-2-methylpropanoic acid::2-[4-(1-hydroxy-4-{4-[hydroxy(diphenyl)methyl]piperidin-1-yl}butyl)phenyl]-2-methylpropanoic acid::Allegra::CHEMBL914::Fexofenadine::MDL-16455
SMILES CC(C)(C(O)=O)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1
InChI Key InChIKey=RWTNPBWLLIMQHL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 22874
Found 4 hits for monomerid = 22874
Affinity DataKi: 27nMAssay Description:Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-7.09kcal/molepH: 7.4 T: 2°CAssay Description:Ligand displacement assays were performed on The SK-N-MC/hH4R cell homogenates. Retained radioactivity was determined by liquid scintillation countin...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataKi: 2.30E+4nMAssay Description:Inhibition of human ERG channelMore data for this Ligand-Target Pair