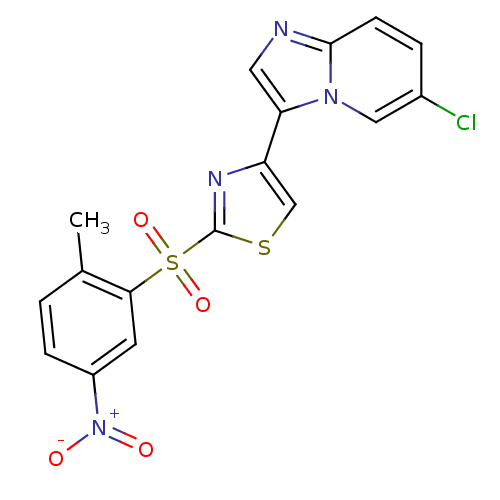
BDBM25056 4-{6-chloroimidazo[1,2-a]pyridin-3-yl}-2-[(2-methyl-5-nitrobenzene)sulfonyl]-1,3-thiazole::imidazo[1,2-a]pyridine derivative, 12
SMILES Cc1ccc(cc1S(=O)(=O)c1nc(cs1)-c1cnc2ccc(Cl)cn12)[N+]([O-])=O
InChI Key InChIKey=WRDUSFKPZVPYTB-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 25056
Found 4 hits for monomerid = 25056
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 2.80nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 100nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 230nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 170nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair