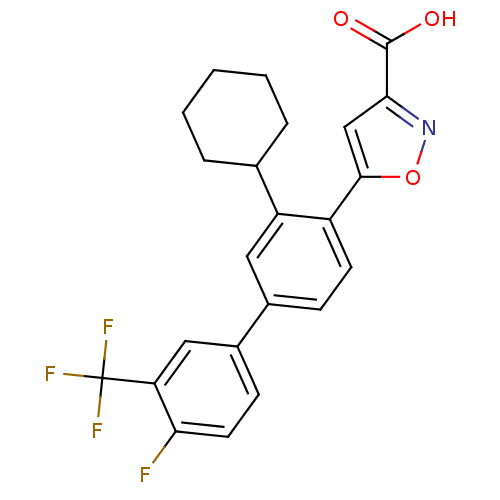
BDBM26104 5-{2-cyclohexyl-4-[4-fluoro-3-(trifluoromethyl)phenyl]phenyl}-1,2-oxazole-3-carboxylic acid::Isoxazole carboxylic acid, 19
SMILES OC(=O)c1cc(on1)-c1ccc(cc1C1CCCCC1)-c1ccc(F)c(c1)C(F)(F)F
InChI Key InChIKey=WDHWIVUIANFBPK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 26104
Found 8 hits for monomerid = 26104
TargetTYR_PHOSPHATASE_2 domain-containing protein(Mycobacterium tuberculosis)
University Of California Berkeley
University Of California Berkeley
Affinity DataKi: 220nM ΔG°: -8.98kcal/molepH: 7.4 T: 2°CAssay Description:The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d...More data for this Ligand-Target Pair
TargetTYR_PHOSPHATASE_2 domain-containing protein(Mycobacterium tuberculosis)
University Of California Berkeley
University Of California Berkeley
Affinity DataKi: 220nMAssay Description:Competitive inhibition of Mycobacterium tuberculosis H37Rv PTP-B expressed in Escherichia coli DE3 using p-nitrophenyl phosphate as substrate preincu...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase C(Homo sapiens (Human))
University Of California Berkeley
University Of California Berkeley
Affinity DataKi: 7.70E+3nM ΔG°: -6.90kcal/molepH: 7.4 T: 2°CAssay Description:The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase F(Homo sapiens (Human))
University Of California Berkeley
University Of California Berkeley
Affinity DataKi: 2.14E+4nM ΔG°: -6.30kcal/molepH: 7.4 T: 2°CAssay Description:The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d...More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nM ΔG°: >-5.80kcal/molepH: 7.4 T: 2°CAssay Description:The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d...More data for this Ligand-Target Pair
TargetLow molecular weight protein-tyrosine phosphatase A(Mycobacterium tuberculosis)
University Of California Berkeley
University Of California Berkeley
Affinity DataKi: >5.00E+4nM ΔG°: >-5.80kcal/molepH: 7.4 T: 2°CAssay Description:The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
University Of California Berkeley
University Of California Berkeley
Affinity DataKi: >5.00E+4nM ΔG°: >-5.80kcal/molepH: 7.4 T: 2°CAssay Description:The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d...More data for this Ligand-Target Pair
TargetTYR_PHOSPHATASE_2 domain-containing protein(Mycobacterium tuberculosis)
University Of California Berkeley
University Of California Berkeley
Affinity DataIC50: 220nMAssay Description:Inhibition of Mycobacterium tuberculosis ptpBMore data for this Ligand-Target Pair