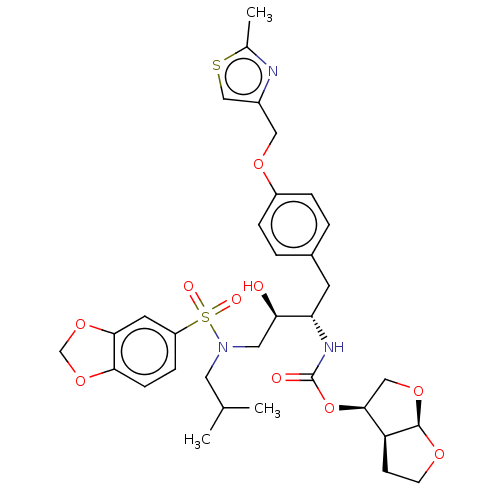
BDBM4685 (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S,3R)-4-[2H-1,3-benzodioxole-5-(2-methylpropyl)sulfonamido]-3-hydroxy-1-{4-[(2-methyl-1,3-thiazol-4-yl)methoxy]phenyl}butan-2-yl]carbamate::CHEMBL206031::GW0385::[3H]GW0385
SMILES [H][C@]12OCC[C@@]1([H])[C@H](CO2)OC(=O)N[C@@H](Cc1ccc(OCc2csc(C)n2)cc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc2OCOc2c1
InChI Key InChIKey=JORVRJNILJXMMG-OLNQLETPSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 4685
Found 8 hits for monomerid = 4685
Affinity DataKi: 0.0000150nM ΔG°: -19.2kcal/molepH: 5.5 T: 2°CAssay Description:The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The...More data for this Ligand-Target Pair
Affinity DataKi: 0.0000150nMAssay Description:Inhibition of HIV1 proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataKi: 0.0000150nMAssay Description:Displacement of [3H]GW0385 from HIV1 proteaseMore data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.000750nM ΔG°: -16.8kcal/molepH: 5.5 T: 2°CAssay Description:The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,I539V](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.00200nM ΔG°: -16.2kcal/molepH: 5.5 T: 2°CAssay Description:The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.00340nM ΔG°: -15.9kcal/molepH: 5.5 T: 2°CAssay Description:The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The...More data for this Ligand-Target Pair
Affinity DataKi: 0.00680nMAssay Description:Inhibition of HIV1 protease dimerization in MT2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.41E+4nMAssay Description:Inhibition of human MDR1-dependent accumulation of calcein-AM expressed in MDCK2 cellsMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)