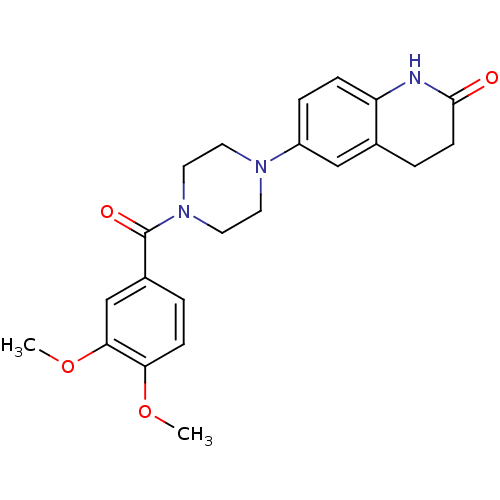
BDBM50016980 6-(4-(3,4-dimethoxybenzoyl)piperazin-1-yl)-3,4-dihydroquinolin-2(1H)-one::6-[4-(3,4-Dimethoxy-benzoyl)-piperazin-1-yl]-3,4-dihydro-1H-quinolin-2-one::6-[4-(3,4-Dimethoxy-benzoyl)-piperazin-1-yl]-3,4-dihydro-1H-quinolin-2-one(OPC-8212)::CHEMBL17423::VESNARINONE::[4-(3,4-Dihydro-quinolin-6-yl)-piperazin-1-yl]-(3,4-dimethoxy-phenyl)-methanone
SMILES COc1ccc(cc1OC)C(=O)N1CCN(CC1)c1ccc2NC(=O)CCc2c1
InChI Key InChIKey=ZVNYJIZDIRKMBF-UHFFFAOYSA-N
Data 9 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50016980
Found 9 hits for monomerid = 50016980
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tcg Lifesciences
Curated by ChEMBL
Tcg Lifesciences
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A(Homo sapiens (Human))
Daqing Oil Field General Hospital
Curated by ChEMBL
Daqing Oil Field General Hospital
Curated by ChEMBL
Affinity DataIC50: 1.12E+4nMAssay Description:Inhibition of human PDE3A using FAM-cAMP by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B(Homo sapiens (Human))
Daqing Oil Field General Hospital
Curated by ChEMBL
Daqing Oil Field General Hospital
Curated by ChEMBL
Affinity DataIC50: 1.45E+4nMAssay Description:Inhibition of human PDE3B using FAM-cAMP by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tcg Lifesciences
Curated by ChEMBL
Tcg Lifesciences
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human Potassium channel HERG expressed in mammalian cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.20E+3nMAssay Description:In vivo inhibition of cyclic AMP phosphodiesterase from human plateletsMore data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A(Homo sapiens (Human))
Daqing Oil Field General Hospital
Curated by ChEMBL
Daqing Oil Field General Hospital
Curated by ChEMBL
Affinity DataIC50: 1.07E+4nMAssay Description:Inhibition of human PDE3A by fluorescence microplate readerMore data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B(Homo sapiens (Human))
Daqing Oil Field General Hospital
Curated by ChEMBL
Daqing Oil Field General Hospital
Curated by ChEMBL
Affinity DataIC50: 1.32E+4nMAssay Description:Inhibition of human PDE3B by fluorescence microplate readerMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tcg Lifesciences
Curated by ChEMBL
Tcg Lifesciences
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human ERG expressed in CHO cells by whole cell patch clamp techniqueMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tcg Lifesciences
Curated by ChEMBL
Tcg Lifesciences
Curated by ChEMBL
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibitory concentration against IKr potassium channelMore data for this Ligand-Target Pair