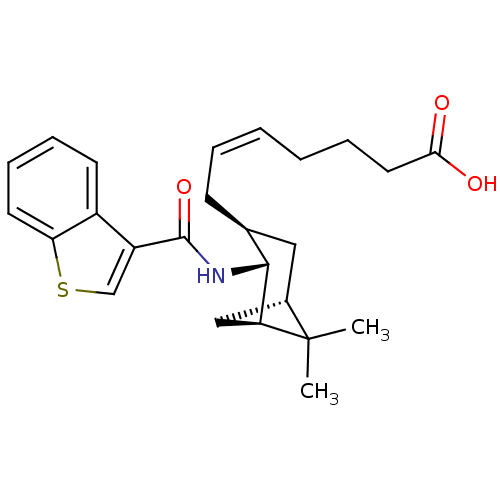
BDBM50060452 (Z)-7-{(1R,2R,3S,5S)-2-[(Benzo[b]thiophene-3-carbonyl)-amino]-6,6-dimethyl-bicyclo[3.1.1]hept-3-yl}-hept-5-enoic acid::CHEMBL332635
SMILES CC1(C)[C@@H]2C[C@H]1[C@H](NC(=O)c1csc3ccccc13)[C@@H](C\C=C/CCCC(O)=O)C2
InChI Key InChIKey=FLLSTOWANOUPLX-JCMHDSIZSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50060452
Found 4 hits for monomerid = 50060452
Affinity DataIC50: 32nMAssay Description:Inhibition of [3H]-PGD-2 specific binding to Prostaglandin D2 receptor fromhuman platelet membranesMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of cAMP formation by carbacyclin in Prostaglandin I2 receptor (IP) assayMore data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:Inhibition of [3H]- (+)-S-145 specific binding to human platelet membranes in TXA2 receptor (TP) assayMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of cAMP formation evoked by the prostaglandin D2 receptor in human plateletsMore data for this Ligand-Target Pair