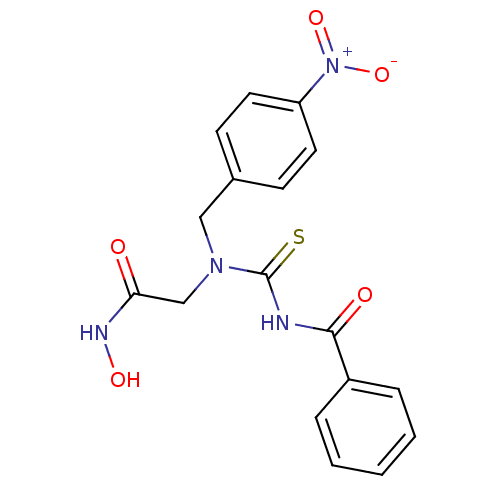
BDBM50088120 2-[3-Benzoyl-1-(4-nitro-benzyl)-thioureido]-N-hydroxy-acetamide::CHEMBL54535
SMILES ONC(=O)CN(Cc1ccc(cc1)[N+]([O-])=O)C(=S)NC(=O)c1ccccc1
InChI Key InChIKey=VURZBHKGMIEEFK-UHFFFAOYSA-N
Data 5 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50088120
Found 5 hits for monomerid = 50088120
Affinity DataKi: 1.40nMAssay Description:Inhibitory activity against the Matrix metalloprotease-9More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibitory activity against the Matrix Metalloprotease-8More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibitory activity against the Matrix Metalloprotease 2 (MMP-2)More data for this Ligand-Target Pair
Target72 kDa type IV collagenase/Collagenase 3/Interstitial collagenase/Matrix metalloproteinase-9/Neutrophil collagenase(Homo sapiens (Human))
Università
Curated by ChEMBL
Università
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Inhibitory activity against the Clostridium histolyticum collagenase (ChC)More data for this Ligand-Target Pair
Affinity DataKi: 37nMAssay Description:Inhibitory activity against the Matrix Metalloprotease 1 (MMP-1)More data for this Ligand-Target Pair