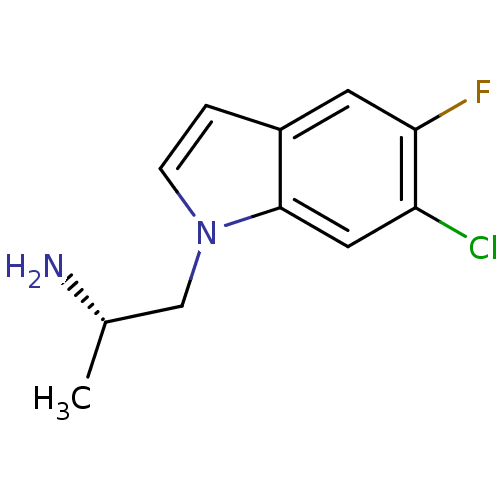
BDBM50144841 (S)-1-(6-chloro-5-fluoro-1H-indol-1-yl)propan-2-amine::(S)-2-(6-Chloro-5-fluoro-indol-1-yl)-1-methyl-ethylamine::CHEMBL76781::Ro-60-0175::Ro-600175
SMILES C[C@H](N)Cn1ccc2cc(F)c(Cl)cc12
InChI Key InChIKey=XJJZQXUGLLXTHO-ZETCQYMHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50144841
Found 3 hits for monomerid = 50144841
Affinity DataKi: 1.30nMAssay Description:Binding affinity against human 5-hydroxytryptamine 2C receptor using displacement of [3H]DOBMore data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Binding affinity against human 5-hydroxytryptamine 2A receptor using displacement of [3H]5-HTMore data for this Ligand-Target Pair
Affinity DataEC50: 200nMAssay Description:Efficacy (pEC50) was evaluated for 5-HT2C receptor-mediated stimulation of IP3 formation in vitro in choroid plexus of the ratMore data for this Ligand-Target Pair