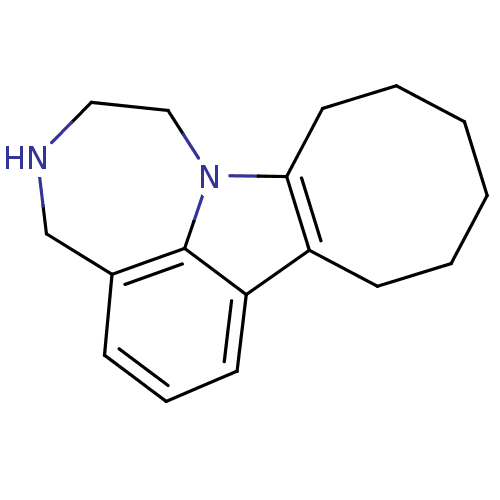
BDBM50145565 1,2,3,4,8,9,10,11,12,13-decahydrocycloocta[b][1,4]diazepino[6,7,1-hi]indole::CHEMBL89738
SMILES C1Cn2c3CCCCCCc3c3cccc(CN1)c23
InChI Key InChIKey=FEOKYCHGJRHIDE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50145565
Found 4 hits for monomerid = 50145565
Affinity DataKi: 13nMAssay Description:Agonistic binding affinity against human 5-hydroxytryptamine 2C receptor in CHO cells using [125I]- DOI More data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:Agonistic binding affinity of the compound against human 5-hydroxytryptamine 2A receptor in CHO cells using 8-OH-DPA radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 665nMAssay Description:Binding affinity of the compound against dopamine receptor D1AMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity of the compound against 5-hydroxytryptamine 1A receptorMore data for this Ligand-Target Pair