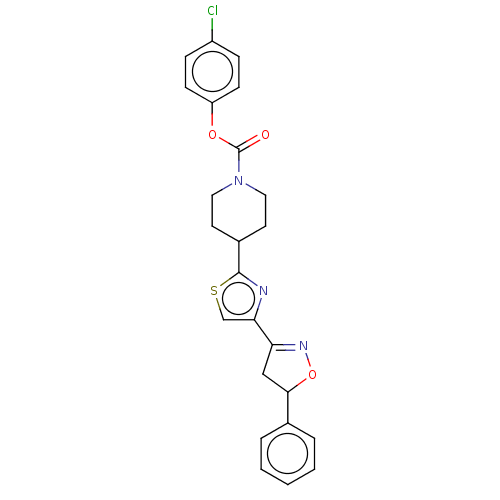
BDBM50166869 CHEMBL3799744
SMILES Clc1ccc(OC(=O)N2CCC(CC2)c2nc(cs2)C2=NOC(C2)c2ccccc2)cc1
InChI Key InChIKey=HXOCNUJNVSGRPA-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50166869
Found 3 hits for monomerid = 50166869
Affinity DataKi: 0.00700nMAssay Description:Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0800nMAssay Description:Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 470nMAssay Description:Inhibition of porcine liver esterase using 4-nitrophenyl butyrate substrate assessed as reduction in p-nitrophenol release measured over 5 minsMore data for this Ligand-Target Pair