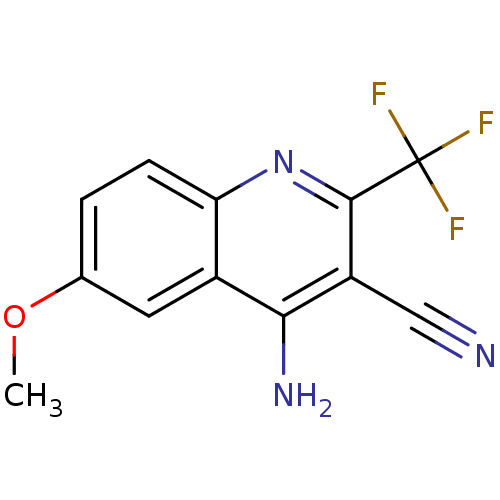
BDBM50210319 4-amino-6-methoxy-2-(trifluoromethyl)quinoline-3-carbonitrile::CHEMBL226970
SMILES COc1ccc2nc(c(C#N)c(N)c2c1)C(F)(F)F
InChI Key InChIKey=RCWHVQVJHLZELL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50210319
Found 3 hits for monomerid = 50210319
TargetATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11(Homo sapiens (Human))
University Of Perugia
Curated by ChEMBL
University Of Perugia
Curated by ChEMBL
Affinity DataEC50: 1.50E+4nMAssay Description:Activity at Kir6.2/SUR1 KATP channels expressed in HEK293 cells assessed as repolarization of tolbutamide-induced membrane depolarizationMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Nanjing University Of Chinese Medicine
Curated by ChEMBL
Nanjing University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 690nMAssay Description:Inhibition of Electrophorus electricus AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.07E+4nMAssay Description:Inhibition of equine serum BChE pre-incubated for 5 mins before butyrylthiocholineiodide substrate addition by Ellman's methodMore data for this Ligand-Target Pair