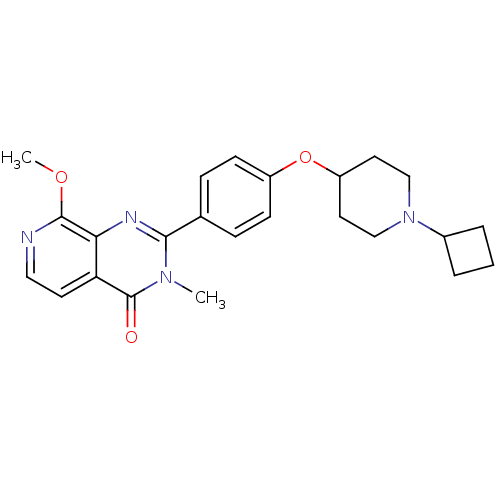
BDBM50246435 2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-methoxy-3-methylpyrido[3,4-d]pyrimidin-4(3H)-one::CHEMBL507360
SMILES COc1nccc2c1nc(-c1ccc(OC3CCN(CC3)C3CCC3)cc1)n(C)c2=O
InChI Key InChIKey=FJPDUNSHNPHJFS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50246435
Found 8 hits for monomerid = 50246435
Affinity DataKi: 0.0938nMAssay Description:Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human histamine H2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human histamine H4 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Displacement of [3H]dofetilide from human recombinant ERG by Competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human histamine H1 receptorMore data for this Ligand-Target Pair