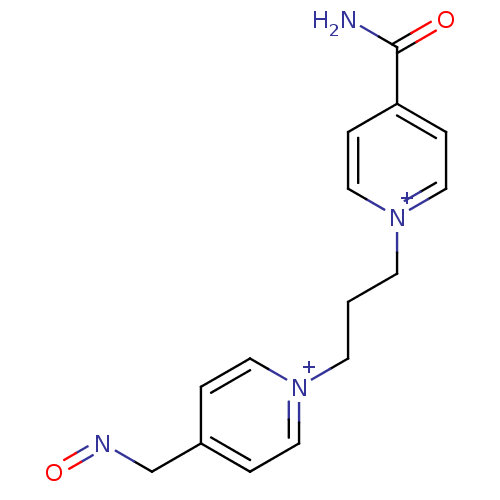
BDBM50333782 4-(aminocarbonyl)-1-(3-{4-[(E)-(hydroxyimino)methyl]pyridinium-1-yl}propyl)pyridinium dibromide::4-carbamoyl-1-(3-(4-((hydroxyimino)methyl)pyridinium-1-yl)propyl)pyridinium bromide::4-carbamoyl-1-(3-(4-((hydroxyimino)methyl)pyridinium-1-yl)propyl)pyridinium dibromide::CHEMBL397871
SMILES NC(=O)c1cc[n+](CCC[n+]2ccc(CN=O)cc2)cc1
InChI Key InChIKey=YTLZKYJTTLZMBF-UHFFFAOYSA-O
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50333782
Found 5 hits for monomerid = 50333782
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 7.30E+4nMAssay Description:Reversible inhibition of human erythrocytic AChE using acetylthiocholine iodide as substrate measured up to 2 mins by spectrophotometric methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 7.30E+4nMAssay Description:Reactivation of OP compound induced inhibition of AChE in human erythrocytes using ATCh as substrate measured up to 2 mins by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 6.60E+5nMAssay Description:Reactivation of tabun induced inhibition of BChE in human plasma using ATCh as substrate by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataIC50: 7.11E+5nMAssay Description:Inhibition of human recombinant AChE by modified Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKd: 4.00E+4nMAssay Description:Binding affinity to paraoxon-inhibited AChE (unknown origin)More data for this Ligand-Target Pair