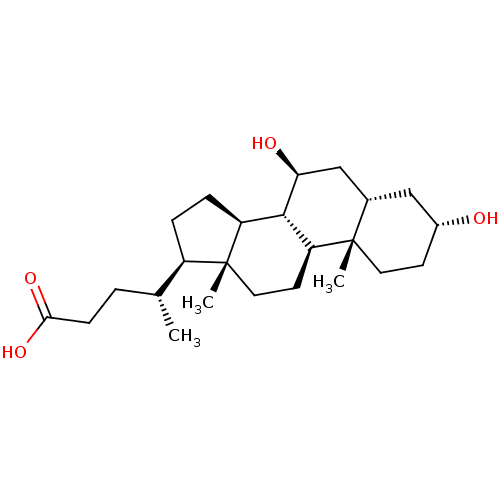
BDBM53721 (4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-10,13-dimethyl-3,7-bis(oxidanyl)-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid::(4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid::(4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]valeric acid::(R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoic acid::3alpha,7beta-dihydroxycholanic acid::Actigall::CHEMBL1551::MLS000028461::SMR000058403::UDCA::URSODEOXYCHOLIC ACID::URSODIOL::Urso 250::Urso forte::Ursodesoxycholic Acid::cid_31401::ursodeoxycholicacid
SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C
InChI Key InChIKey=RUDATBOHQWOJDD-UZVSRGJWSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 17 hits for monomerid = 53721
Found 17 hits for monomerid = 53721
West Virginia University
Curated by ChEMBL
Wake Forest University
Curated by ChEMBL
The Beckman Research Institute
Curated by ChEMBL
Centre National de la Recherche Scientifique/INSERM/ULP
Curated by ChEMBL
The Beckman Research Institute
Curated by ChEMBL
Vanderbilt University School Of Medicine
Curated by ChEMBL
West Virginia University
Curated by ChEMBL
Centre National de la Recherche Scientifique/INSERM/ULP
Curated by ChEMBL
The Beckman Research Institute
Curated by ChEMBL
Srmlsc
Curated by PubChem BioAssay
Jagiellonian University
 3D Structure (crystal)
3D Structure (crystal)