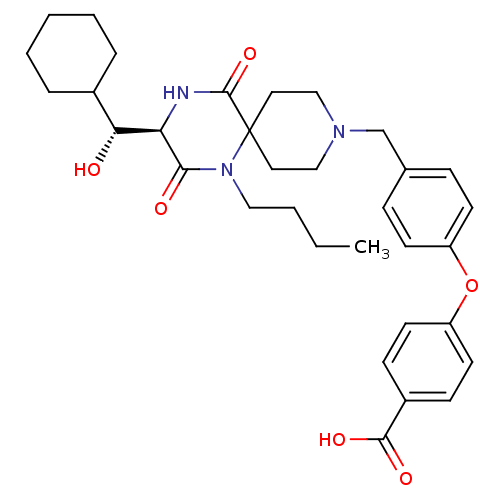
BDBM50336345 4-(4-(((R)-1-butyl-3-((R)-cyclohexyl(hydroxy)methyl)-2,5-dioxo-1,4,9-triazaspiro[5.5]undecan-9-yl)methyl)phenoxy)benzoic acid hydrochloride::APLAVIROC::CHEMBL1668019
SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@H](O)C1CCCCC1
InChI Key InChIKey=GWNOTCOIYUNTQP-FQLXRVMXSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 50336345
Found 15 hits for monomerid = 50336345
Affinity DataIC50: 5.62nMAssay Description:Antagonist activity at human CCR5 expressed in HOS cells assessed as inhibition of cell fusion with HIV gp120 expressing HEK293 cells by LTR lucifera...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Displacement of [125I]-RANTES from CCR5 in mouse NIH/3T3 cells after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization Ca assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.80nMAssay Description:Displacement of MIP-1alpha from human CCR5 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.80nMAssay Description:Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced chemotaxisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Antagonist activity at CXCR4 assessed as inhibition of SDF1-induced calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataIC50: 46nMAssay Description:Antagonist activity at human CCR5 assessed as inhibition of RANTES-induced calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Antagonist activity at rabbit CCR5 assessed as inhibition of RANTES-induced calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataIC50: 5.60E+3nMAssay Description:Antagonist activity against human CCR5 receptor assessed as inhibition of HIV1 gp120-induced cell-cell fusion between viral envolop protein expressin...More data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+4nMAssay Description:Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Antagonist activity at rat CCR5 assessed as inhibition of RANTES-induced calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair