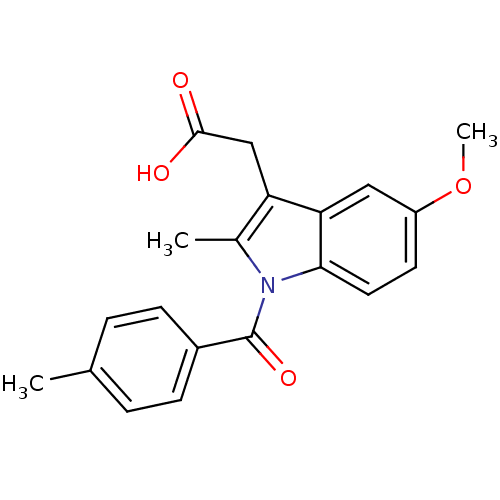
BDBM50427629 CHEMBL179587::US9346803, Table 2, Compound 7: 2-[5-methoxy-2-methyl-1-(4-methylbenzoyl)-1H-indol-3-yl]acetic acid
SMILES COc1ccc2n(C(=O)c3ccc(C)cc3)c(C)c(CC(O)=O)c2c1
InChI Key InChIKey=ASIGZZOVSCNZDW-UHFFFAOYSA-N
Data 8 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50427629
Found 8 hits for monomerid = 50427629
Affinity DataIC50: 0.160nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: >100nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 54.5nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Inhibition of human recombinant AKR1C3-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphtholMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C4(Homo sapiens (Human))
Vanderbilt University School Of Medicine
Curated by ChEMBL
Vanderbilt University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human recombinant AKR1C4-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphtholMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human recombinant AKR1C1-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphtholMore data for this Ligand-Target Pair
Affinity DataIC50: 5.45E+4nMAssay Description:Inhibition of human recombinant AKR1C2-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphtholMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C4(Homo sapiens (Human))
Vanderbilt University School Of Medicine
Curated by ChEMBL
Vanderbilt University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: >100nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair