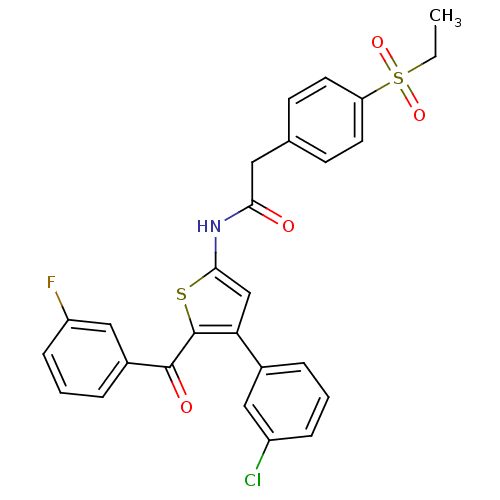
BDBM50445875 CHEMBL3105671
SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2cc(c(s2)C(=O)c2cccc(F)c2)-c2cccc(Cl)c2)cc1
InChI Key InChIKey=XXPNMHPZWJXACX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50445875
Found 3 hits for monomerid = 50445875
Affinity DataKi: 32nMAssay Description:Displacement of 25-[26,27-3H]hydroxycholesterol from RORgammat receptor ligand binding domain (unknown origin) after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of APC-labeled RORgammat receptor ligand binding domain (unknown origin) after 1 hr by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of RORgammat receptor ligand binding domain in mouse spleen CD4+ T cells assessed as inhibition of IL-17 production after 3 days by ELISAMore data for this Ligand-Target Pair