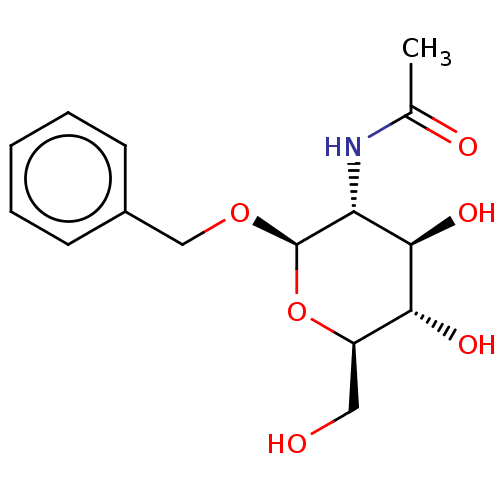
BDBM50466400 CHEMBL1818656
SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OCc1ccccc1
InChI Key InChIKey=SKOZFDIGKDPQBO-KJWHEZOQSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50466400
Found 3 hits for monomerid = 50466400
TargetPoly-beta-1,6-N-acetyl-D-glucosamine N-deacetylase(Escherichia coli (Enterobacteria))
University Of Toronto
Curated by ChEMBL
University Of Toronto
Curated by ChEMBL
Affinity DataIC50: >2.00E+7nMAssay Description:Inhibition of Escherichia coli PgaB expressed in Escherichia coli BL21 (DE3) cells transformed with pET28 plasmid coding for PgaB42-655 using acetoxy...More data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Rattus norvegicus)
Jiangsu Institute Of Nuclear Medicine
Curated by ChEMBL
Jiangsu Institute Of Nuclear Medicine
Curated by ChEMBL
Affinity DataKd: 4.81E+4nMAssay Description:Binding affinity to rat FPPS expressed in Escherichia coli BL21 (DE3) by isothermal titration calorimetryMore data for this Ligand-Target Pair
TargetPeptidoglycan-N-acetylglucosamine deacetylase(Streptococcus pneumoniae (Firmicutes))
University Of Toronto
Curated by ChEMBL
University Of Toronto
Curated by ChEMBL
Affinity DataIC50: 1.10E+7nMAssay Description:Inhibition of Streptococcus pneumoniae Pgda expressed in Escherichia coli BL21 (DE3) cells transformed with pET28bSpPgdA232-431 plasmid using acetoxy...More data for this Ligand-Target Pair