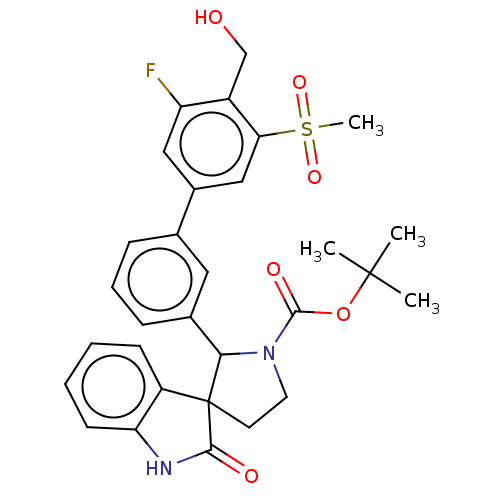
BDBM50551951 CHEMBL4749385
SMILES CC(C)(C)OC(=O)N1CCC2(C1c1cccc(c1)-c1cc(F)c(CO)c(c1)S(C)(=O)=O)C(=O)Nc1ccccc21
InChI Key InChIKey=HYQGRYRHNUHTJF-UHFFFAOYSA-N
Data 2 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50551951
Found 2 hits for monomerid = 50551951
TargetOxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataEC50: 0.103nMAssay Description:Agonist activity at human RXRalpha/LXRbeta expressed in HEK293 cells measured after 20 hrs by dual luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataEC50: 0.251nMAssay Description:Agonist activity at human RXRalpha/LXRalpha expressed in HEK293 cells measured after 20 hrs by dual luciferase reporter gene assayMore data for this Ligand-Target Pair