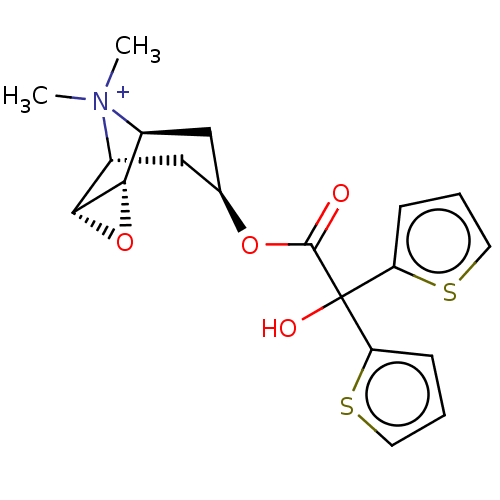
BDBM50581209 CHEMBL4650755
SMILES [H][C@]12O[C@@]1([H])[C@]1([H])C[C@H](C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1
InChI Key InChIKey=LERNTVKEWCAPOY-VOGVJGKGSA-N
Data 2 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50581209
Found 2 hits for monomerid = 50581209
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.126nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.158nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M2 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair