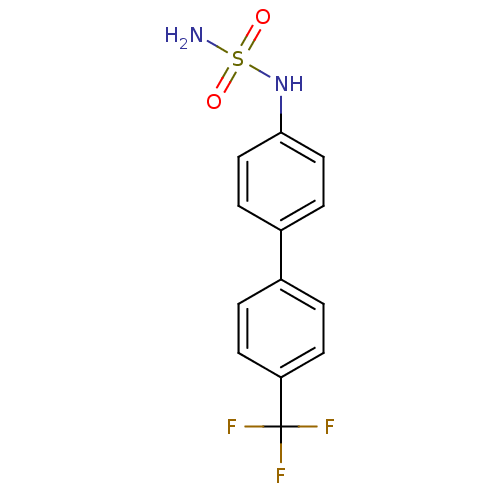
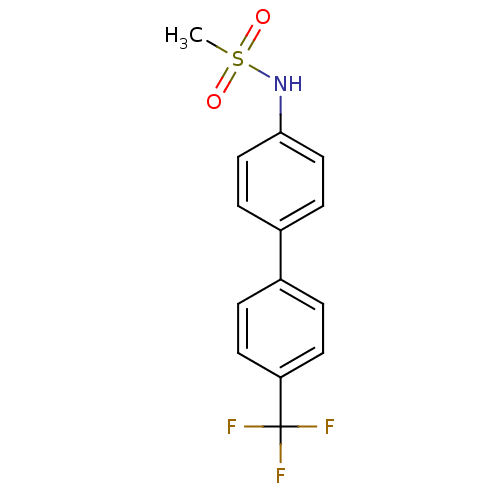
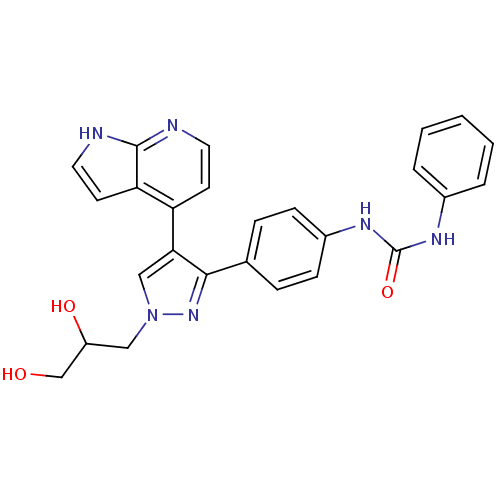
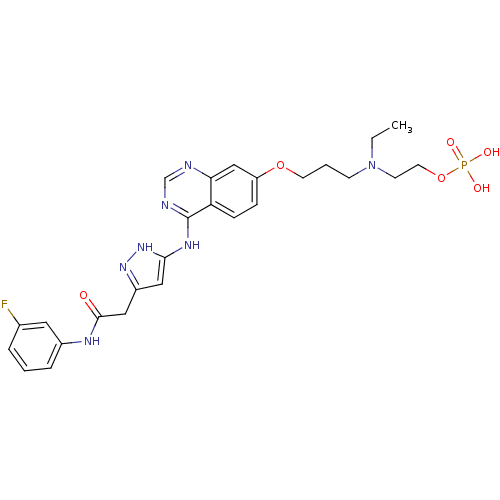
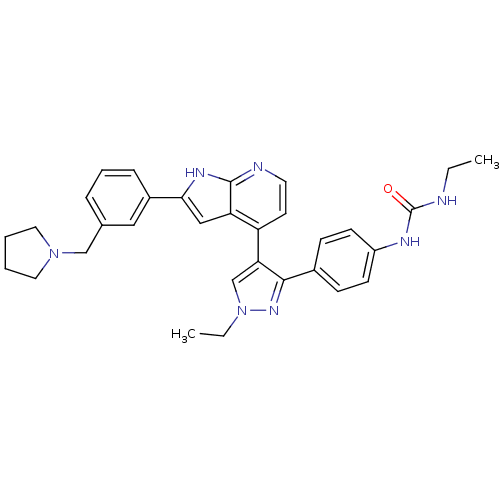
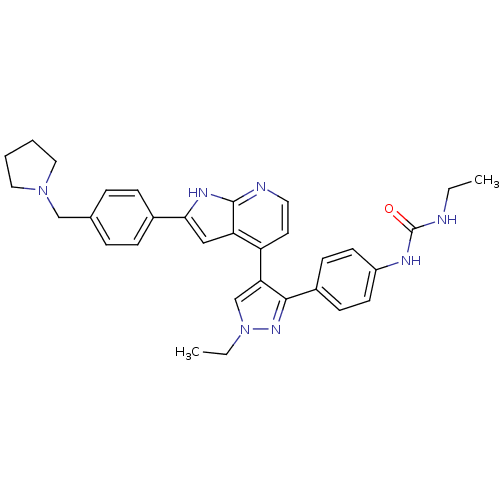
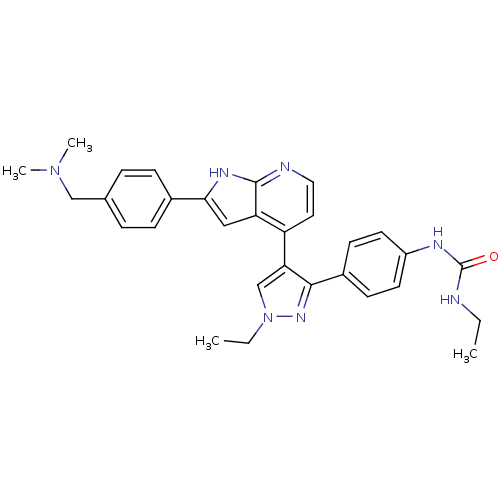
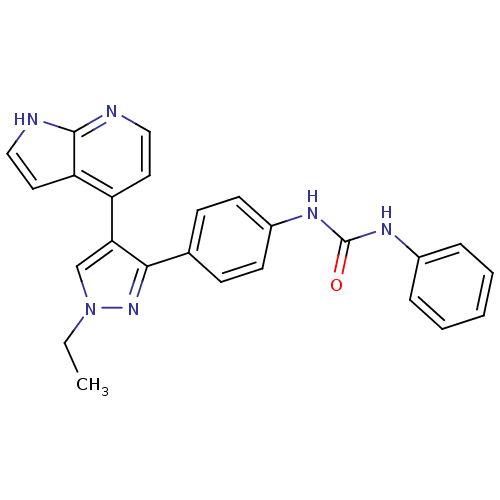
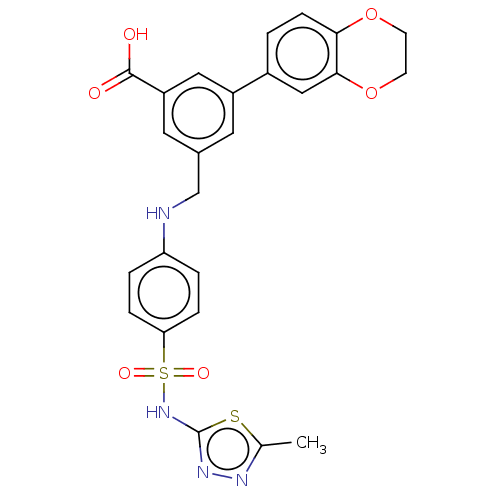
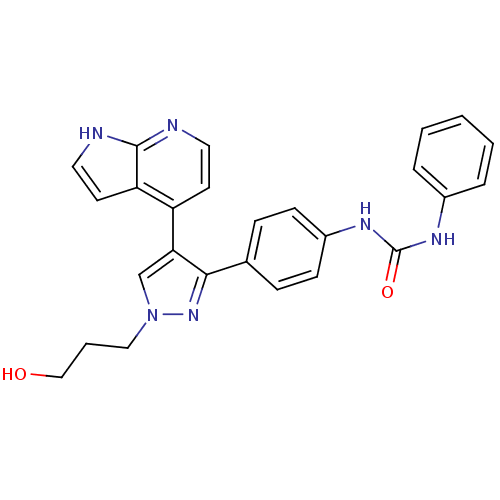
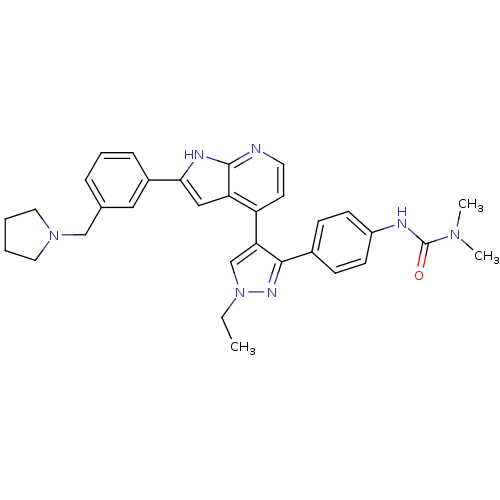
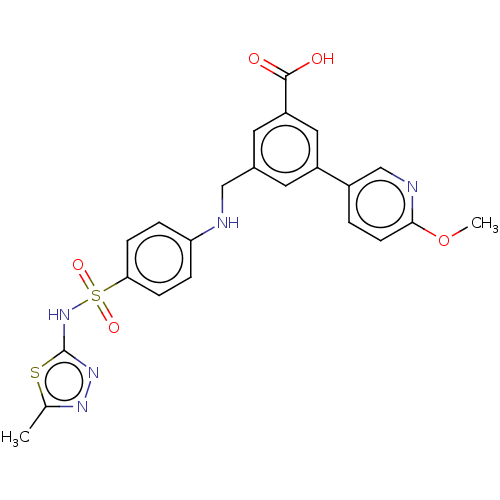
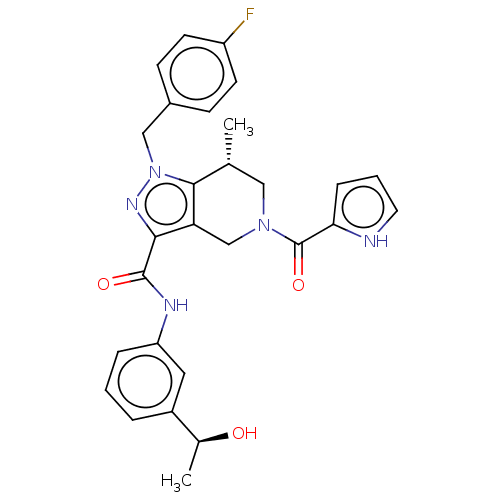
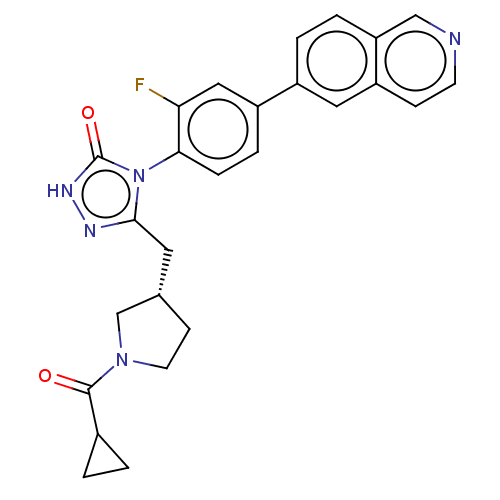
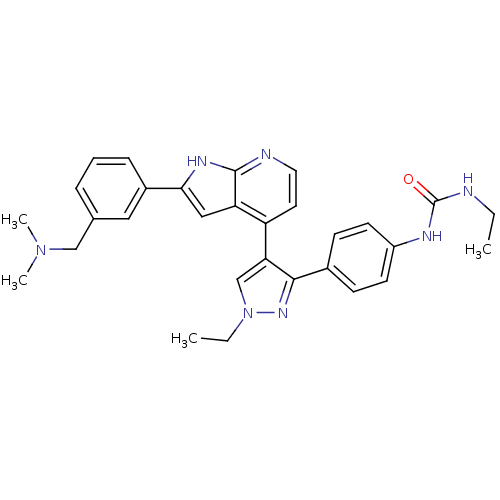
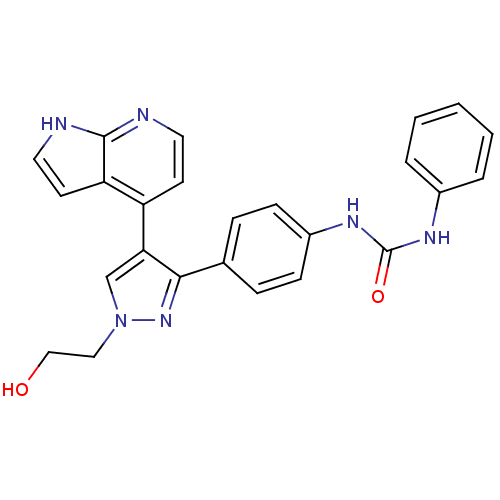
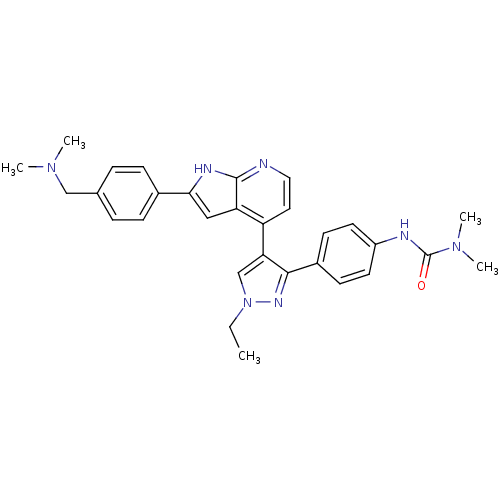
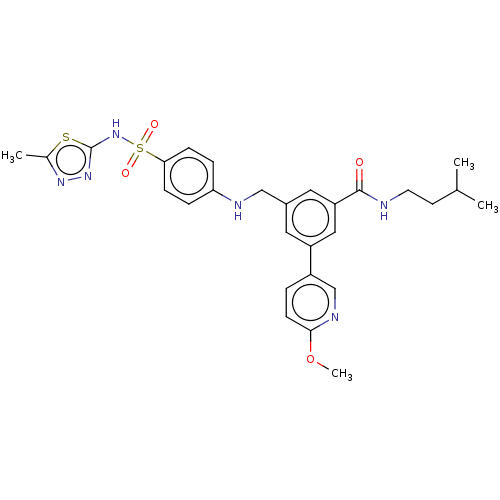
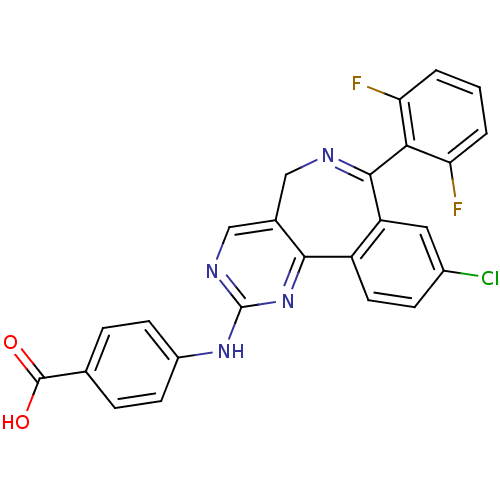
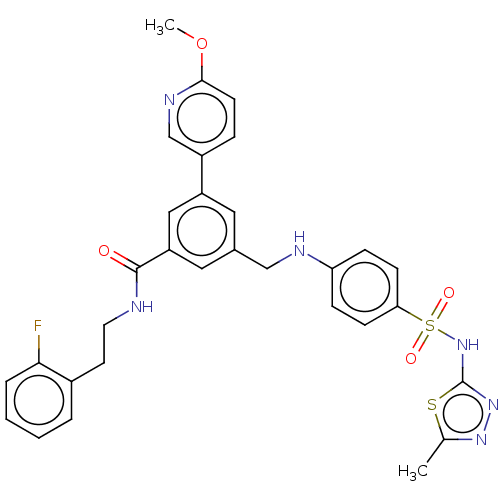
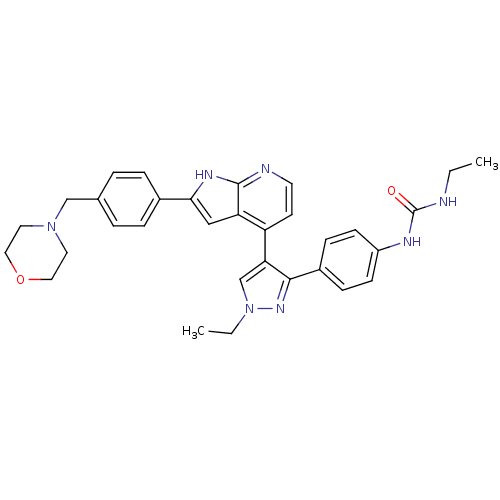
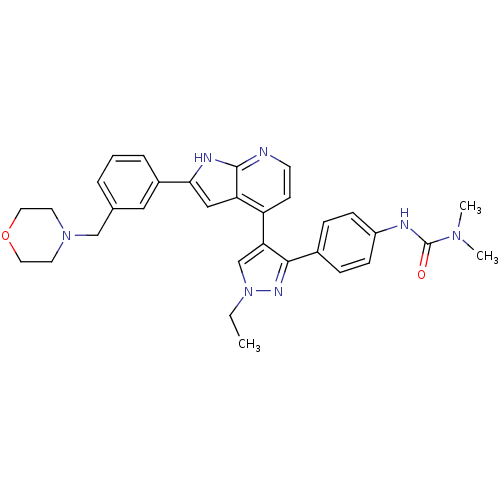
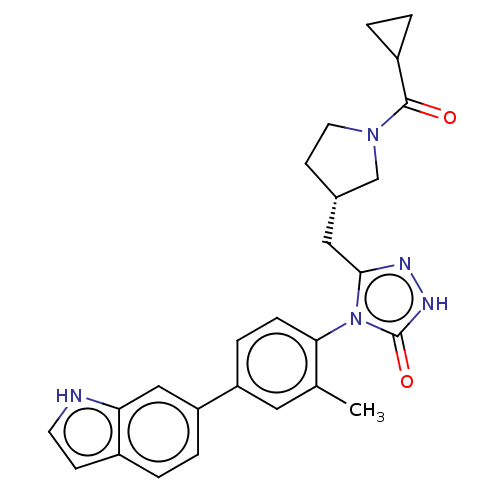
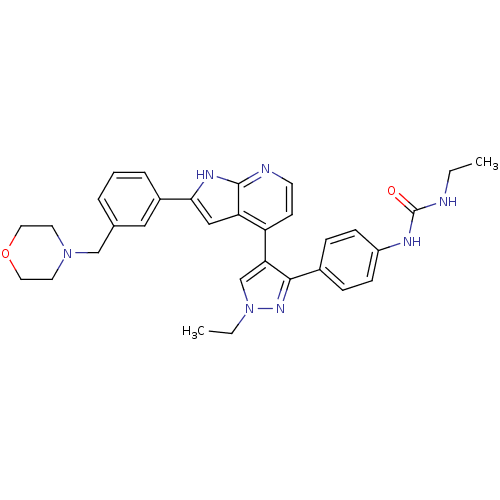
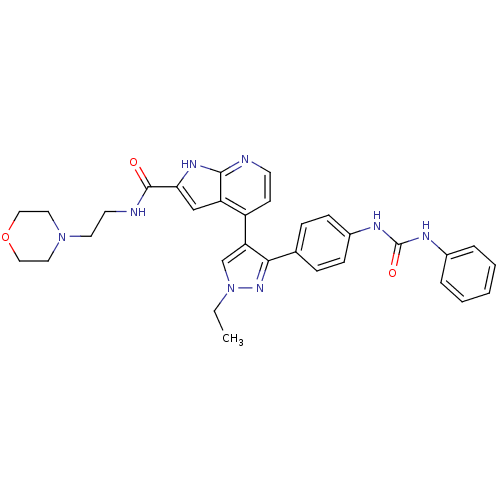
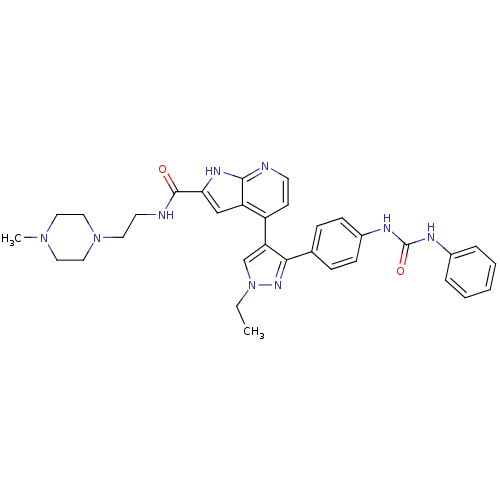
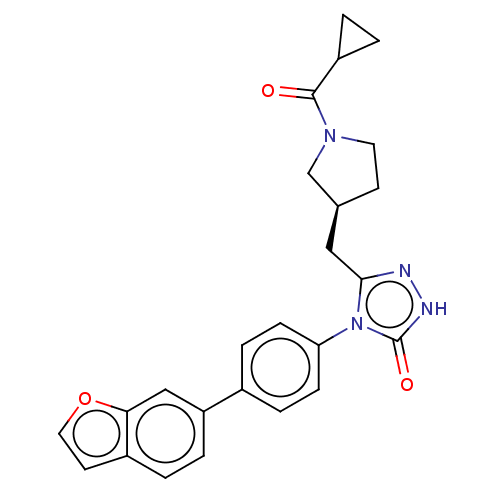
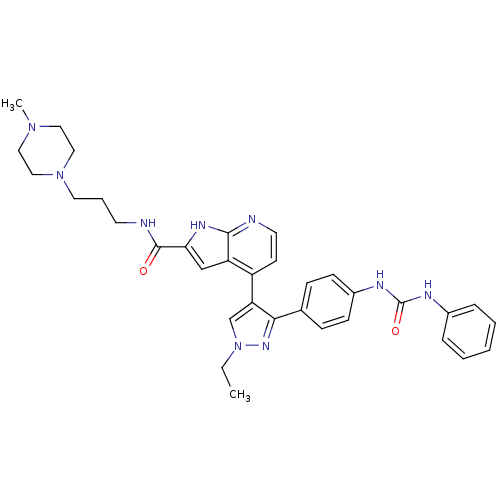
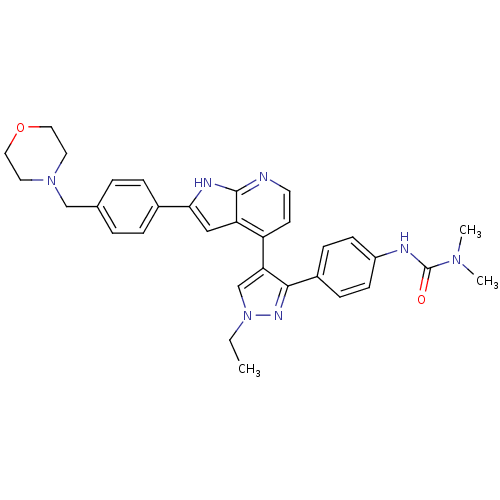
Affinity DataKi: 0.380nMAssay Description:Competitive inhibition of Aurora B ATP binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 0.380nMAssay Description:Competitive inhibition of human Aurora B ATP binding site by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Inhibition of Aurora AMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Competitive inhibition of human Aurora C ATP binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Competitive inhibition of Aurora B ATP binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 3.5nMAssay Description:Inhibition of human KSP motor domain by ATPase assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.60nMAssay Description:Competitive inhibition of Aurora C ATP binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 6.20nMAssay Description:Inhibition of human KSP motor domain by ATPase assayMore data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Inhibition of human KSP motor domain by ATPase assayMore data for this Ligand-Target Pair
Affinity DataKi: 490nMAssay Description:Competitive inhibition of human Aurora A ATP binding siteMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Competitive inhibition of Aurora B ATP binding siteMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of PI3Kgamma (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Inhibition of PI3Kgamma (unknown origin)More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132G](Homo sapiens (Human))
Albert Einstein College Of Medicine
Albert Einstein College Of Medicine
Affinity DataIC50: 2.90nMT: 2°CAssay Description:RapidFire-MS/MS measurements of mutant IDH1 and IDH2 reactions were conducted at room temperature in 384-well Greiner polypropylene microtiter plates...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.0Assay Description:Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132C](Homo sapiens (Human))
Albert Einstein College Of Medicine
Albert Einstein College Of Medicine
Affinity DataIC50: 3.80nMT: 2°CAssay Description:RapidFire-MS/MS measurements of mutant IDH1 and IDH2 reactions were conducted at room temperature in 384-well Greiner polypropylene microtiter plates...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of PI3Kgamma (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of PI3Kdelta (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 4.40nMAssay Description:Inhibition of PI3Kgamma (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Albert Einstein College Of Medicine
Albert Einstein College Of Medicine
Affinity DataIC50: 4.60nMT: 2°CAssay Description:RapidFire-MS/MS measurements of mutant IDH1 and IDH2 reactions were conducted at room temperature in 384-well Greiner polypropylene microtiter plates...More data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human KSP motor domain by ATPase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.0Assay Description:Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.0Assay Description:Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is...More data for this Ligand-Target Pair
Affinity DataIC50: 6.5nMAssay Description:Competitive inhibition of Aurora C ATP binding siteMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 6.5nMAssay Description:Inhibition of wild type his-tagged PI3Kalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.10nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 8.5nMAssay Description:Inhibition of PI3Kalpha H1047R mutant (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 9.60nMAssay Description:Inhibition of wild type his-tagged PI3Kalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.0Assay Description:Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of PI3Kdelta (unknown origin)More data for this Ligand-Target Pair

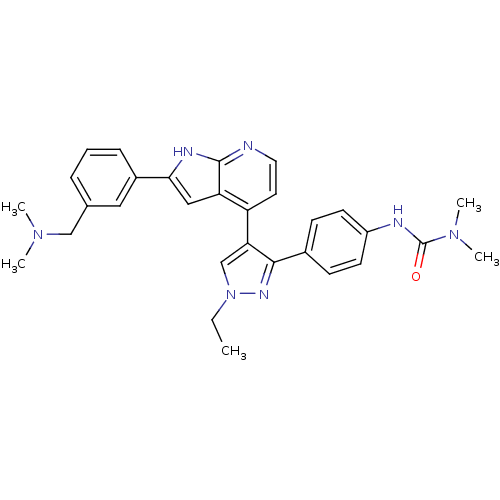
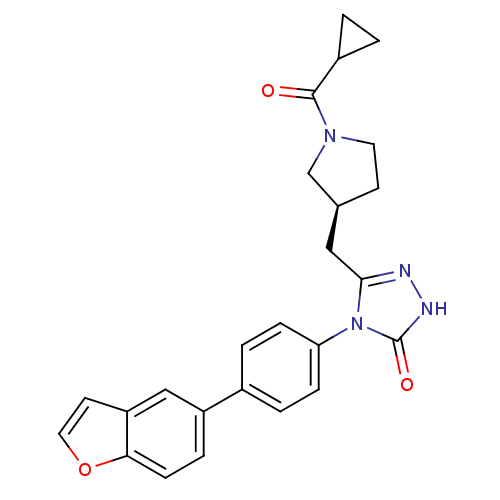
 3D Structure (crystal)
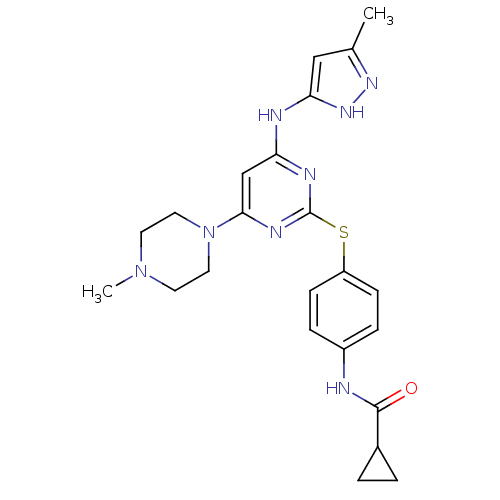
3D Structure (crystal)