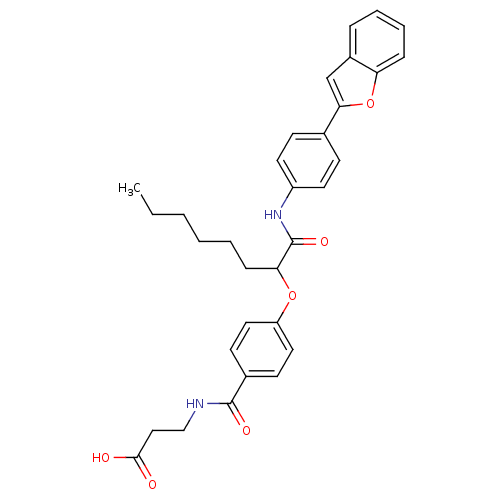
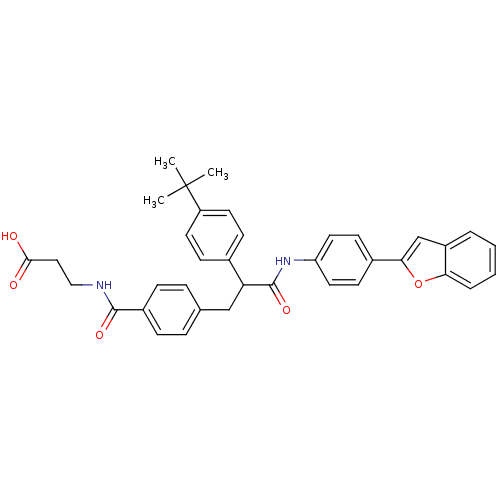
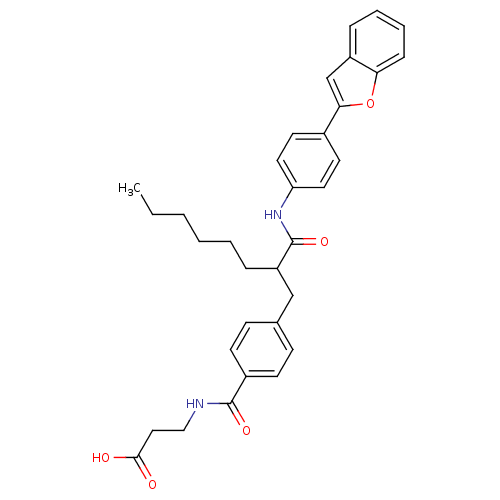
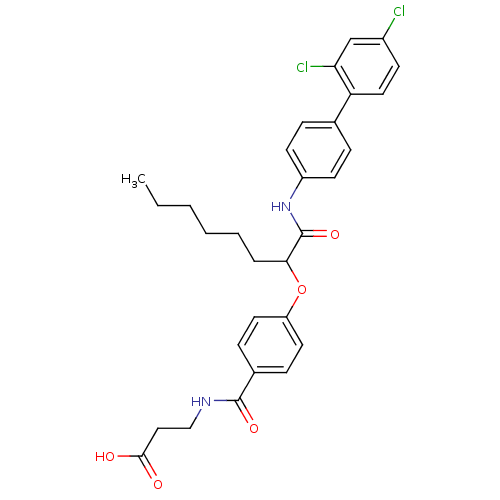
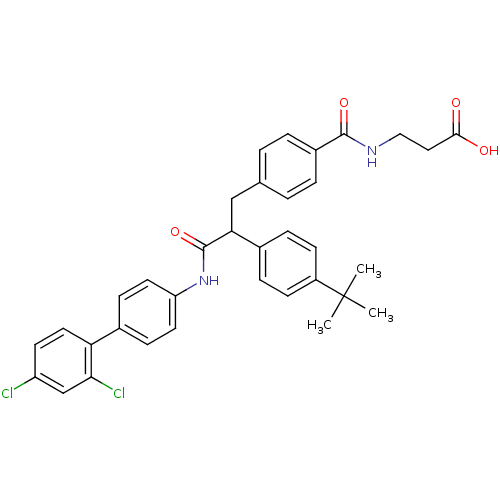
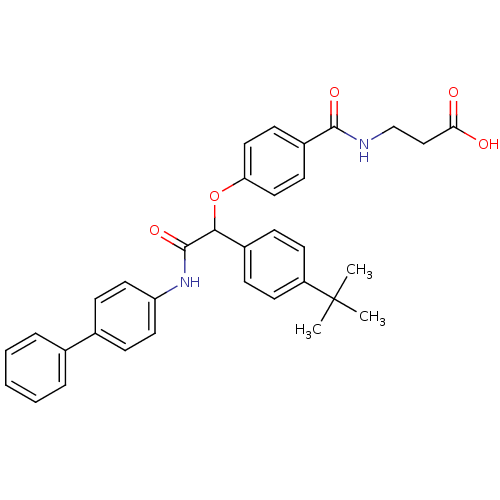
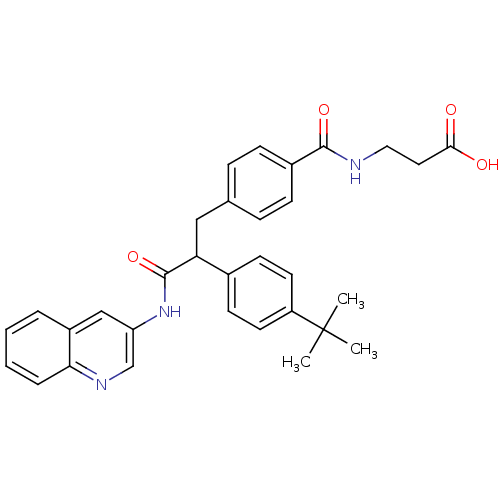
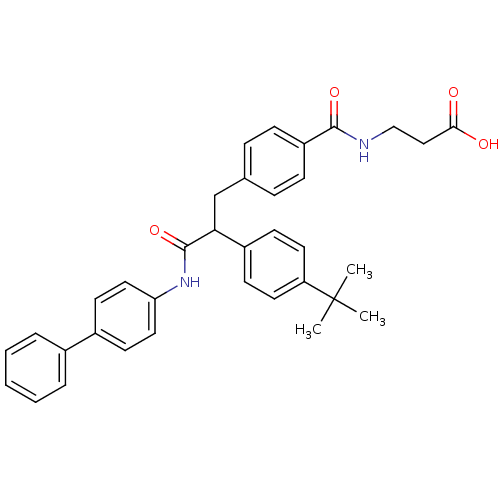
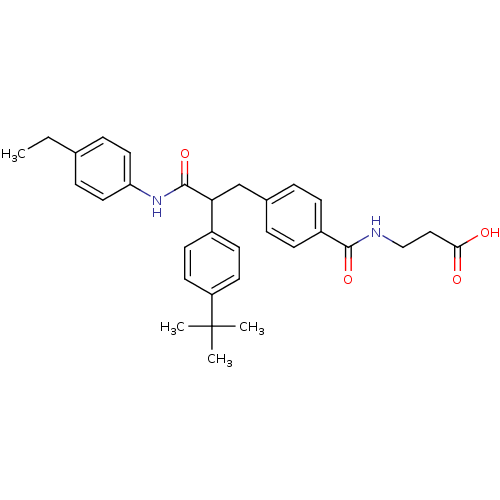
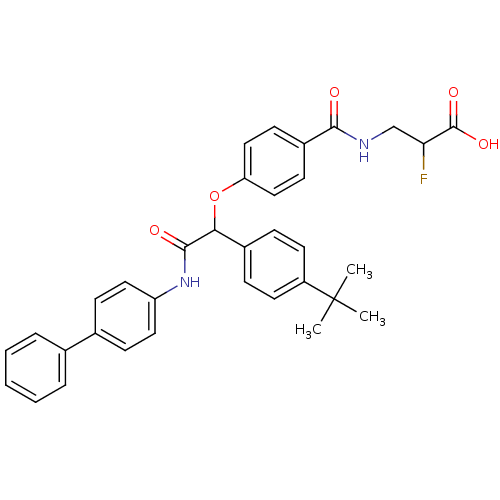
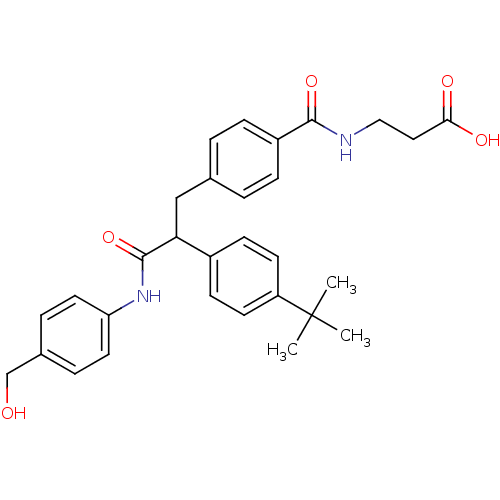
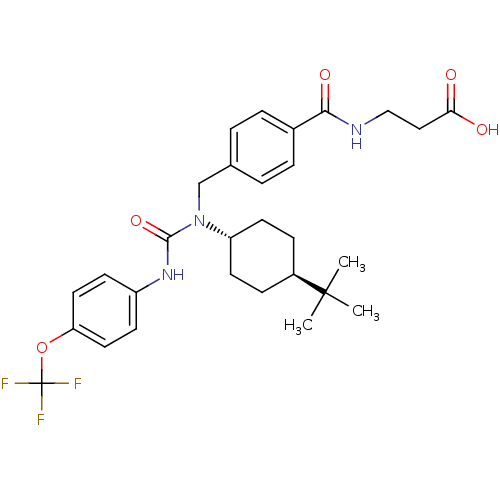
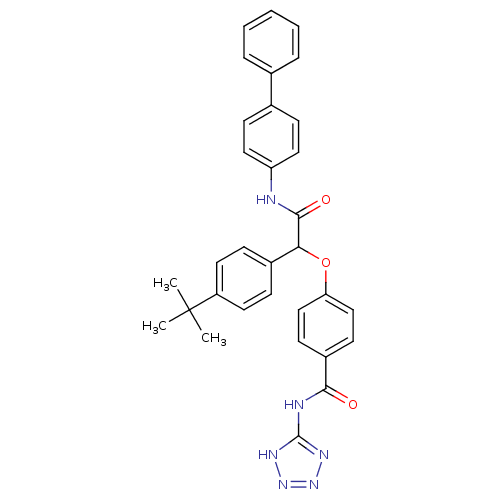
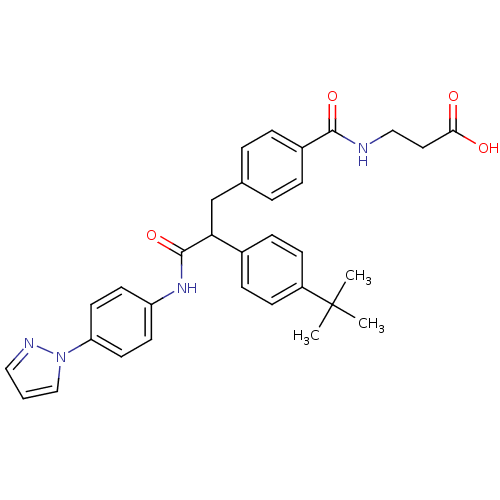
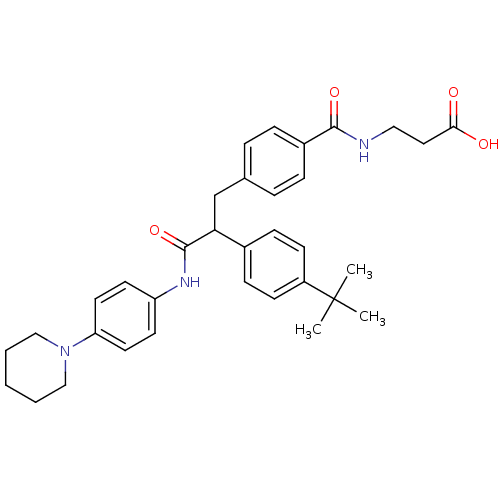
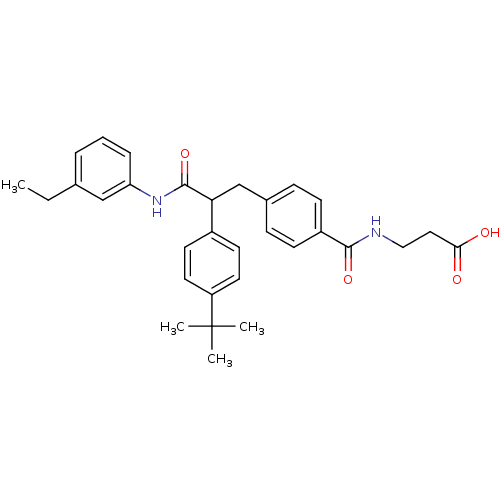
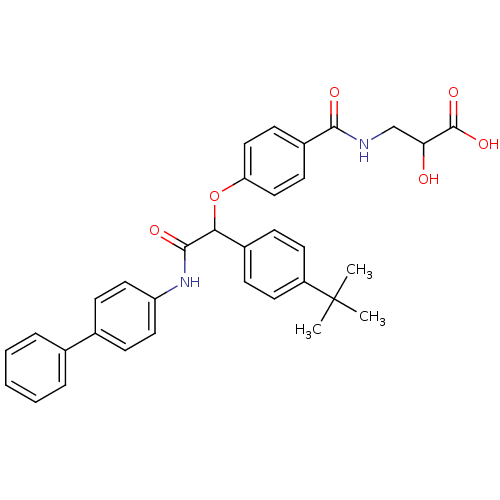
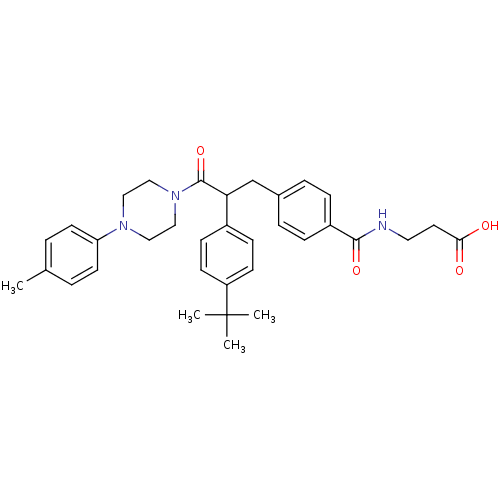
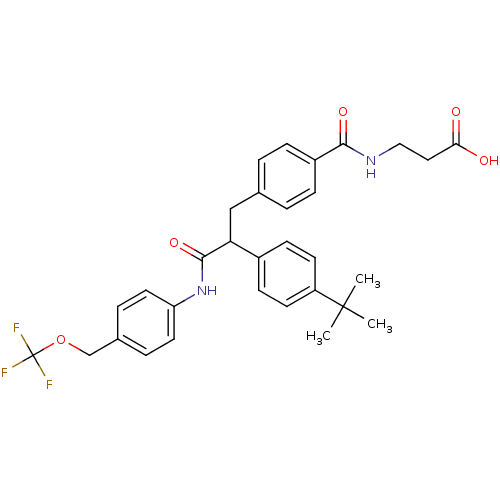
Affinity DataKi: 3nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 29nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 33nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
Affinity DataKi: 51nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 63nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 66nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 69nMAssay Description:In vitro inhibitory activity against glucagon induced monkey adenylate cyclaseMore data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 73nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 110nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
Affinity DataKi: 112nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 144nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 254nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 300nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 317nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 610nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 5.00E+3nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair