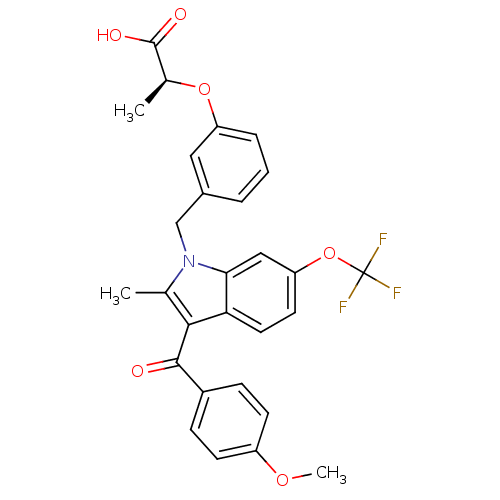
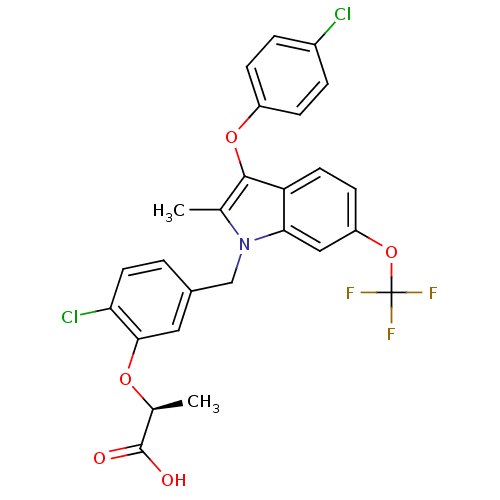
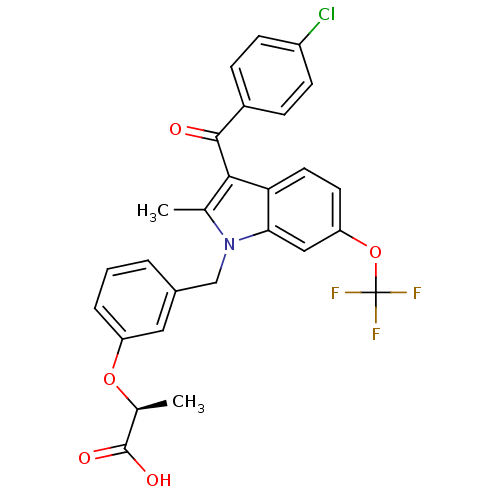
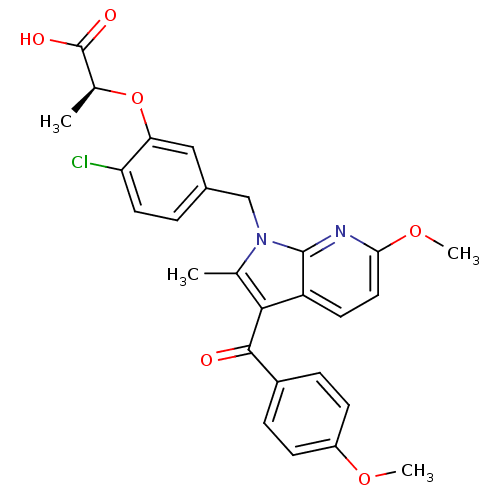
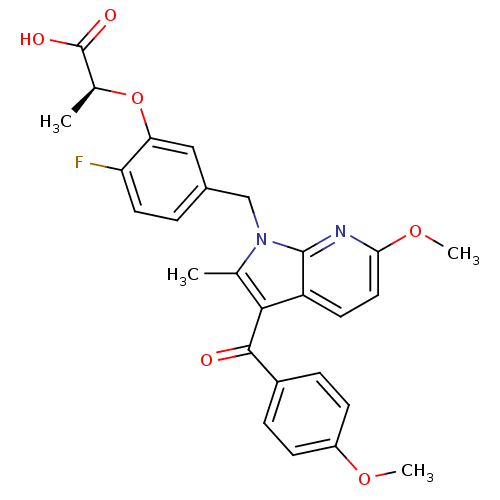
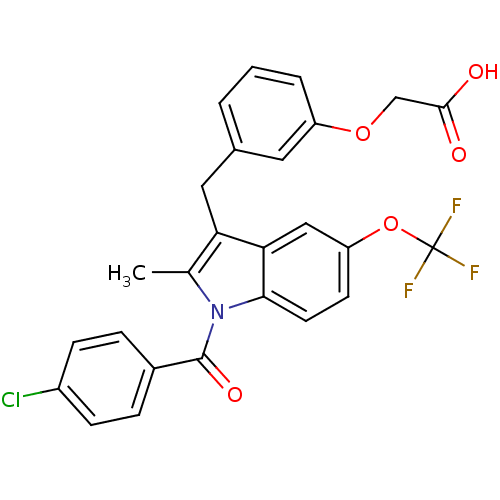
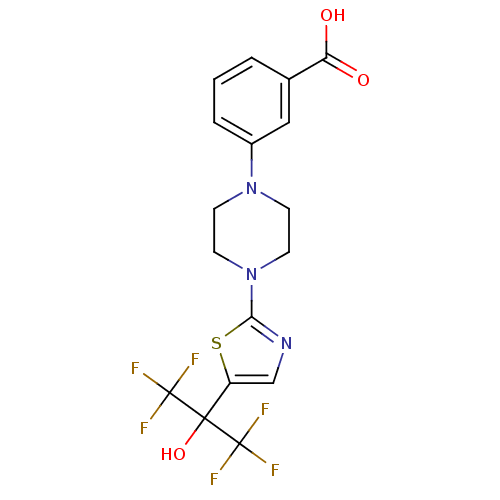
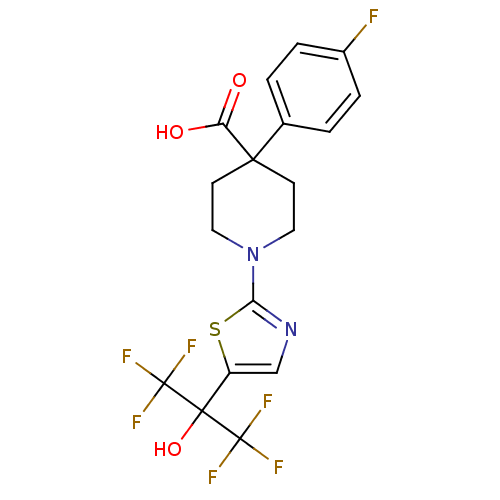
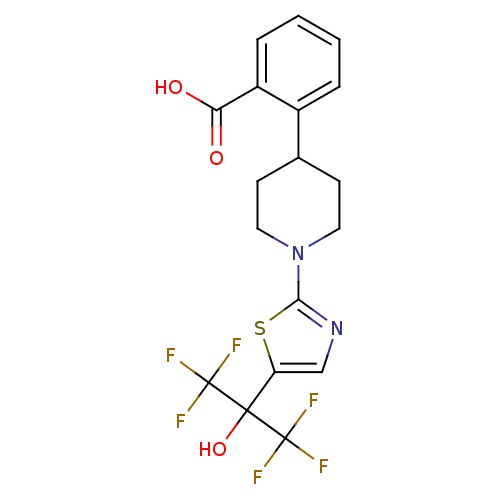
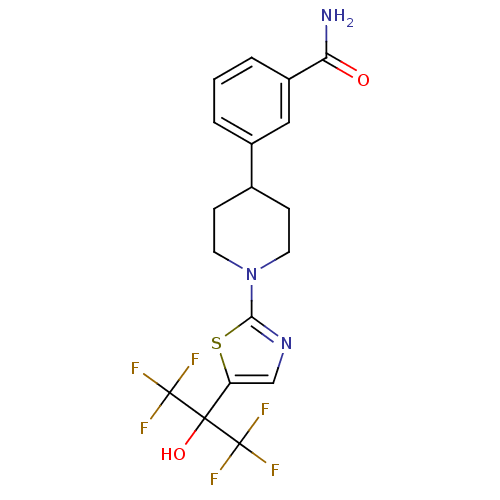
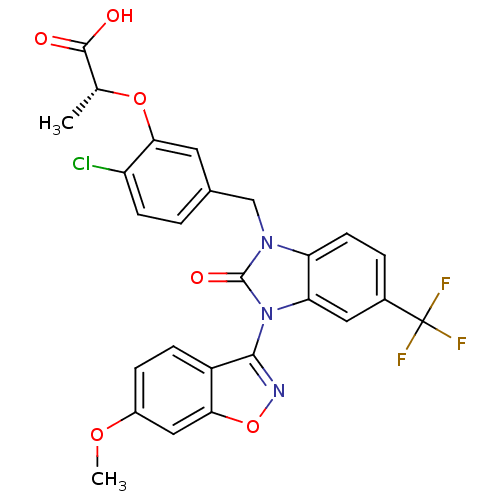
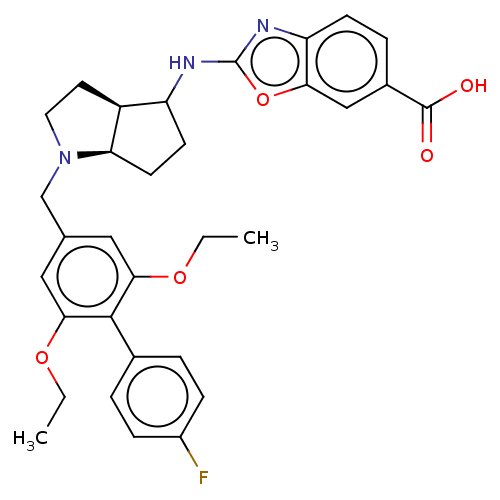
TargetGastrin/cholecystokinin type B receptor(RAT)
University Of Occupational And Environmental Health
Curated by PDSP Ki Database
University Of Occupational And Environmental Health
Curated by PDSP Ki Database
TargetGastrin/cholecystokinin type B receptor(RAT)
University Of Occupational And Environmental Health
Curated by PDSP Ki Database
University Of Occupational And Environmental Health
Curated by PDSP Ki Database
TargetGastrin/cholecystokinin type B receptor(RAT)
University Of Occupational And Environmental Health
Curated by PDSP Ki Database
University Of Occupational And Environmental Health
Curated by PDSP Ki Database
TargetGastrin/cholecystokinin type B receptor(RAT)
University Of Occupational And Environmental Health
Curated by PDSP Ki Database
University Of Occupational And Environmental Health
Curated by PDSP Ki Database
TargetGastrin/cholecystokinin type B receptor(RAT)
University Of Occupational And Environmental Health
Curated by PDSP Ki Database
University Of Occupational And Environmental Health
Curated by PDSP Ki Database
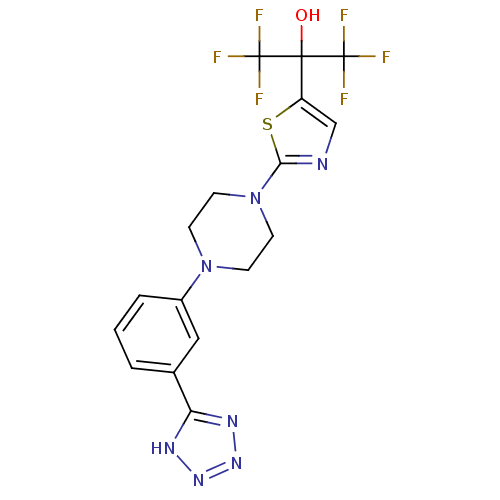
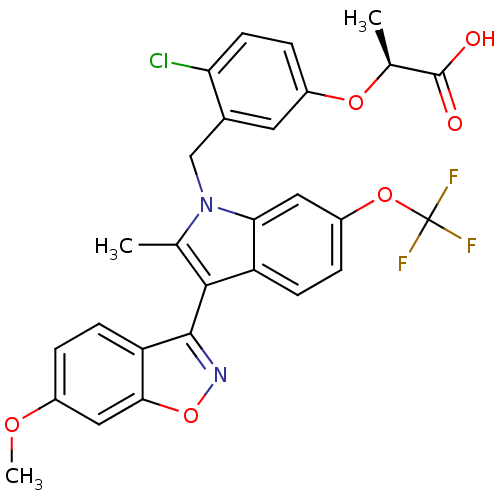
TargetSphingomyelin phosphodiesterase 2(Homo sapiens (Human))
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataKi: 1.60E+3nMAssay Description:Inhibitory activity of the compound against schyphostatin of neutral sphingomyelinase (N-SMase) from bovine brain microsomeMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(RAT)
University Of Occupational And Environmental Health
Curated by PDSP Ki Database
University Of Occupational And Environmental Health
Curated by PDSP Ki Database
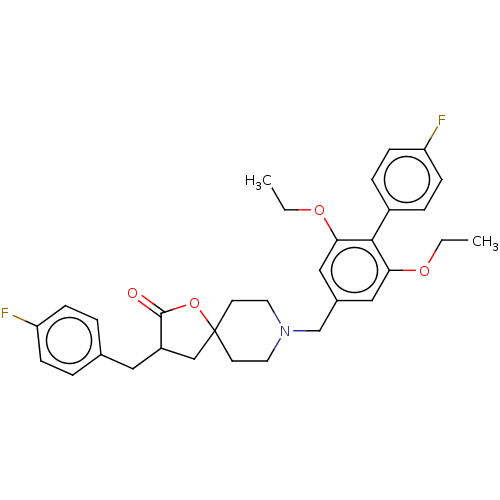
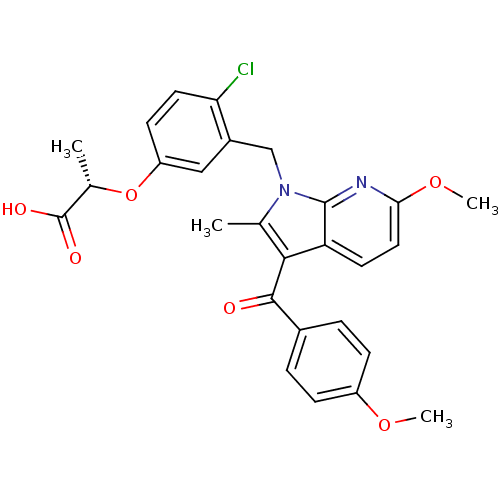
TargetUbiquitin carboxyl-terminal hydrolase 5(Homo sapiens (Human))
Tokyo University Of Agriculture
Curated by ChEMBL
Tokyo University Of Agriculture
Curated by ChEMBL
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of wild type human USP5 expressed in Escherichia coli BL21(DE3) using Ub-AMC as substrate after 60 mins by fluorometric analysisMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(RAT)
University Of Occupational And Environmental Health
Curated by PDSP Ki Database
University Of Occupational And Environmental Health
Curated by PDSP Ki Database
TargetUbiquitin carboxyl-terminal hydrolase 5(Homo sapiens (Human))
Tokyo University Of Agriculture
Curated by ChEMBL
Tokyo University Of Agriculture
Curated by ChEMBL
Affinity DataKi: 1.25E+4nMAssay Description:Competitive inhibition of wild type human USP5 expressed in Escherichia coli BL21(DE3) using Ub-AMC as substrateMore data for this Ligand-Target Pair
TargetSphingomyelin phosphodiesterase 2(Homo sapiens (Human))
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University Of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataKi: 2.53E+5nMAssay Description:Inhibitory activity against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair
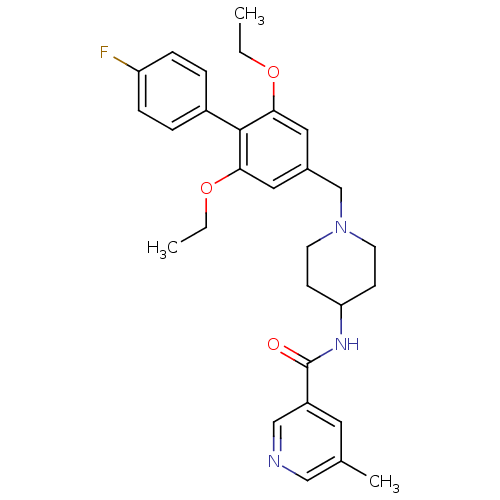
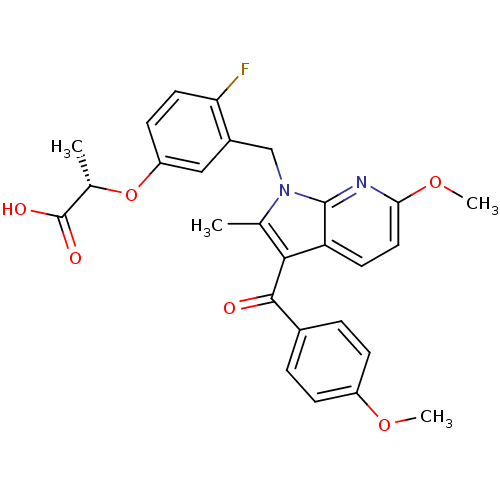
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assayMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assayMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Antagonist activity at mouse SSTR5 expressed in CHO-K1 cell membranes assessed as reduction in SST-28-induced inhibition of forskolin-stimulated cAMP...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human PPARgamma receptor by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human PPARgamma receptor by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Antagonist activity at human SSTR5 expressed in CHO-K1 cell membranes assessed as reduction in SST-28-induced inhibition of forskolin-stimulated cAMP...More data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assayMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assayMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human PPARgamma receptor by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibitory concentration against human peroxisome proliferator activated receptor gamma in SPA assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assayMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.20nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Binding affinity to human PPARgamma by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assayMore data for this Ligand-Target Pair

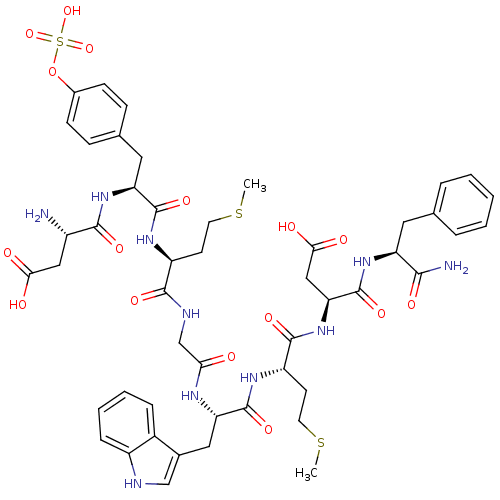
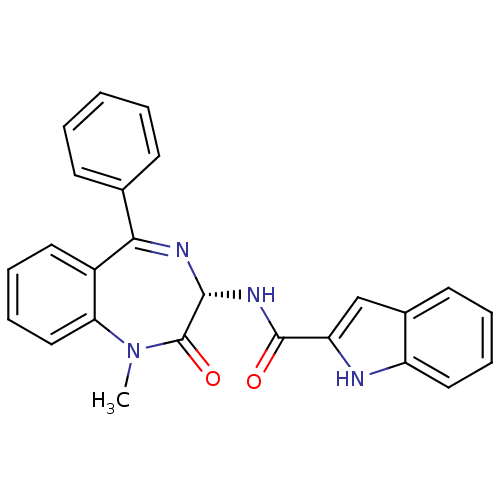
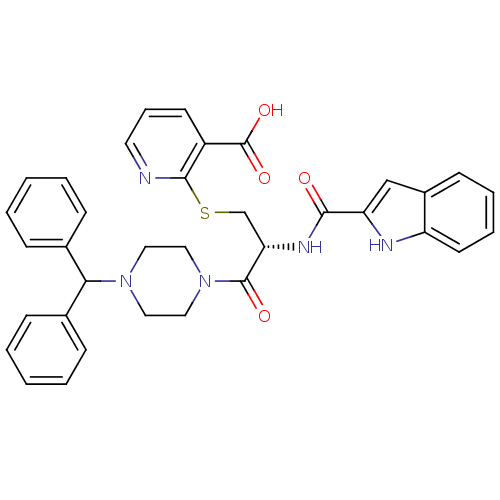
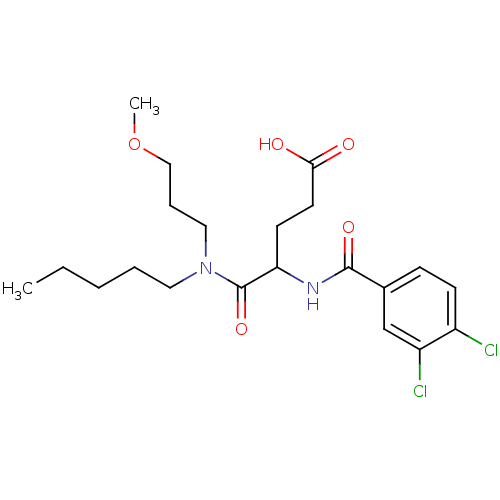
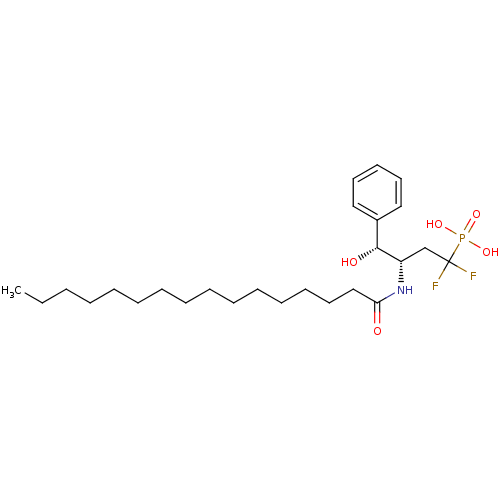
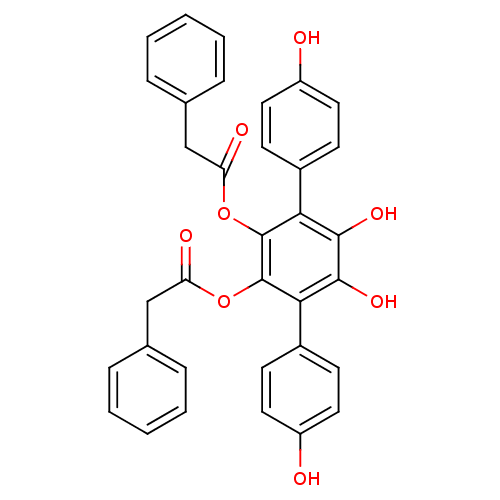
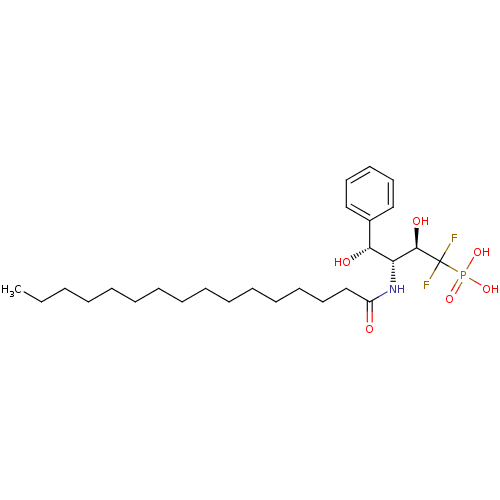
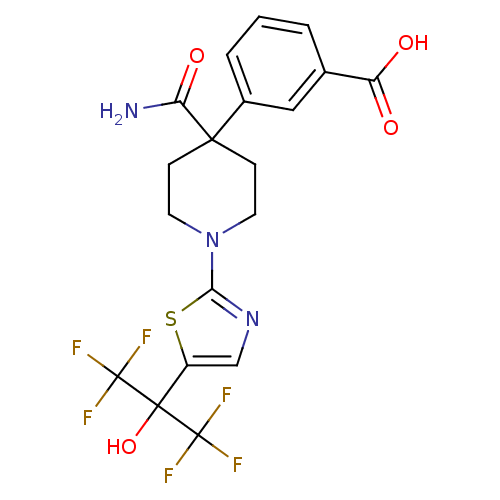
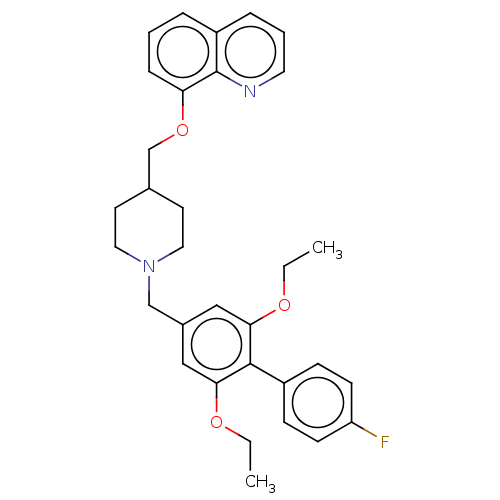
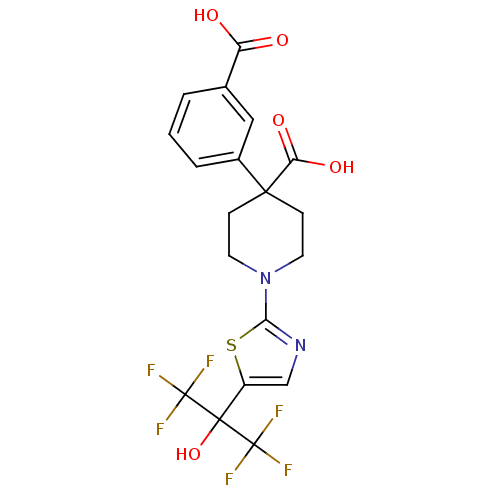



 3D Structure (crystal)
3D Structure (crystal)