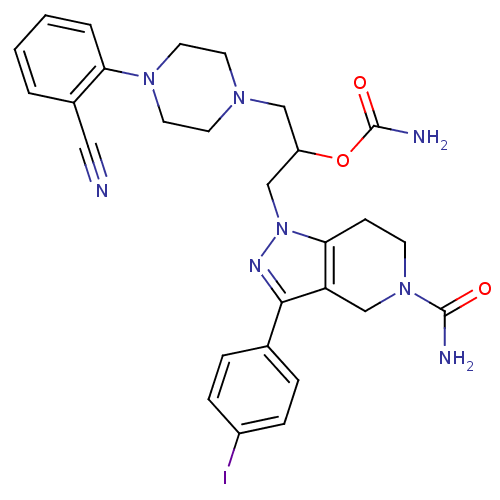
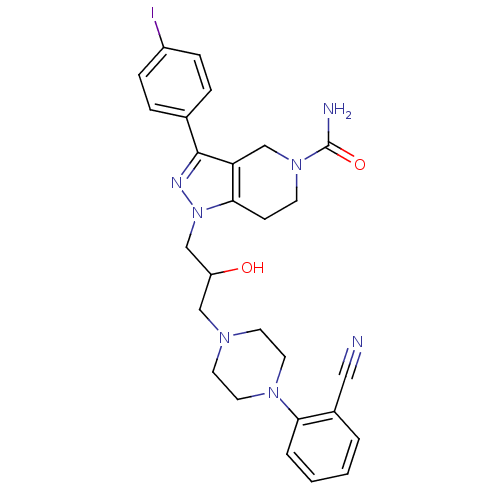
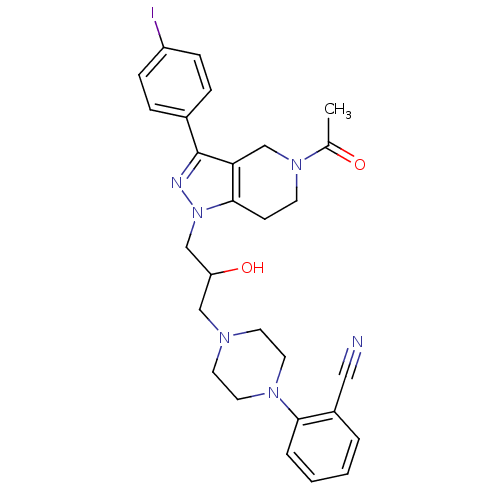
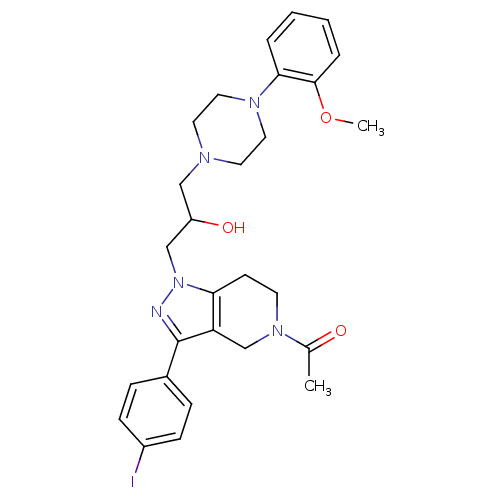
TargetCathepsin S(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
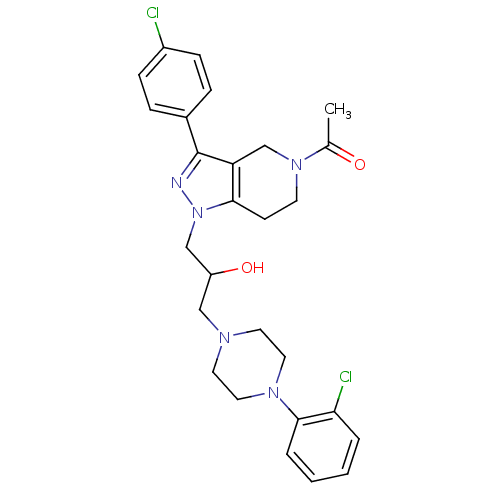
Affinity DataIC50: 5nMAssay Description:Inhibitory concentration against human cysteine protease cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
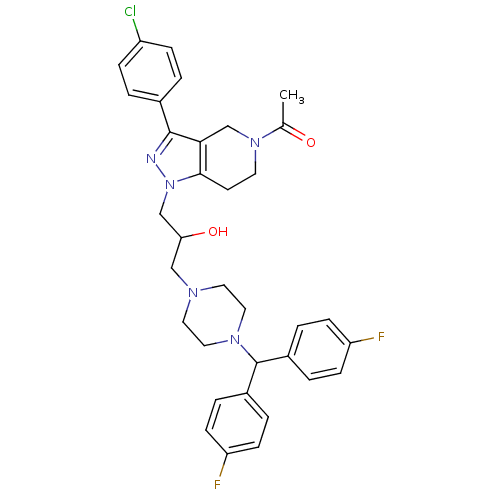
Affinity DataIC50: 20nMAssay Description:Inhibitory concentration against human cysteine protease cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
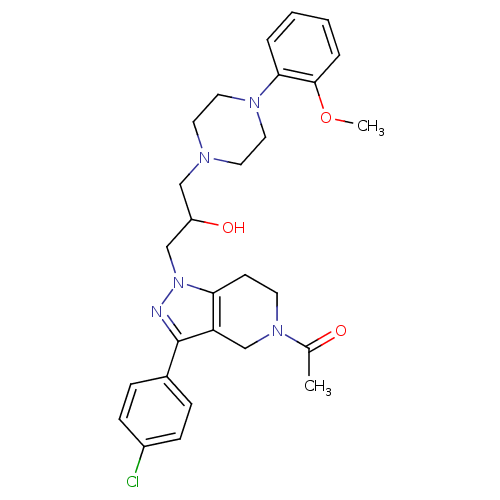
Affinity DataIC50: 50nMAssay Description:Inhibitory concentration against human cysteine protease cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
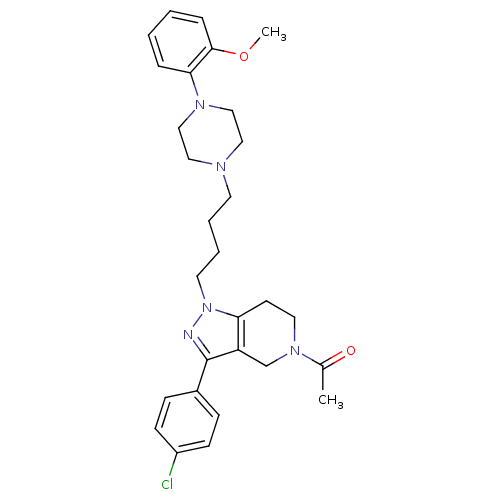
Affinity DataIC50: 120nMAssay Description:Inhibitory concentration against human cysteine protease cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibitory concentration against human cysteine protease cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory concentration against human cysteine protease cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory concentration against human cysteine protease cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibitory concentration against human cysteine protease cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 9.50E+3nMAssay Description:Inhibitory concentration against human cysteine protease cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibitory concentration against human cysteine protease cathepsin SMore data for this Ligand-Target Pair